Chapter19: Aldehydes And Ketones: Nucleophilic Addition Reactions
Section19.SE: Something Extra
Problem 55AP: Draw and name the seven aldehydes and ketones with the formula C5H10O. Which are chiral?
Related questions
Question
4-Cyanophenol has a pKa = 8.0, whereas phenol has a pKa= 10.0. Resonance form that best illustrates why 4-cyanophenol is more acidic is
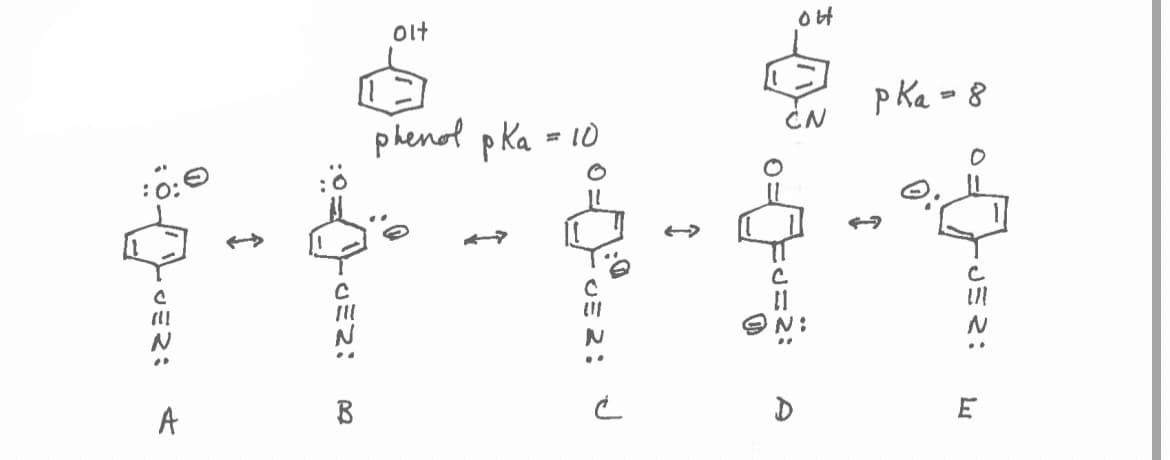
Transcribed Image Text:JER:
A
JER:
C
B
Olt
phenol pka = 10
J
←
OH
CN
:2=ں:
A
рка - 8
-JER:
E
Expert Solution
Step 1
4-Cyanophenol has a pKa = 8.0, whereas phenol has a pKa= 10.0
We have to explain why 4-Cyanophenol is more acidic.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning