4 Part B If you dilute 17.0 mL of the stock solution to a final volume of 0.300 L, what will be the concentration of the diluted solution? Express the molarity in moles per liter to three significant figures. ΜΕ ΑΣΦ Molarity= Submit Request Answer ? M
4 Part B If you dilute 17.0 mL of the stock solution to a final volume of 0.300 L, what will be the concentration of the diluted solution? Express the molarity in moles per liter to three significant figures. ΜΕ ΑΣΦ Molarity= Submit Request Answer ? M
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter3: Mass Relations In Chemistry; Stoichiometry
Section: Chapter Questions
Problem 76QAP: Acetic acid (HC2H3O2) can be prepared by the action of the acetobacter organism on dilute solutions...
Related questions
Question
Help me answer part b
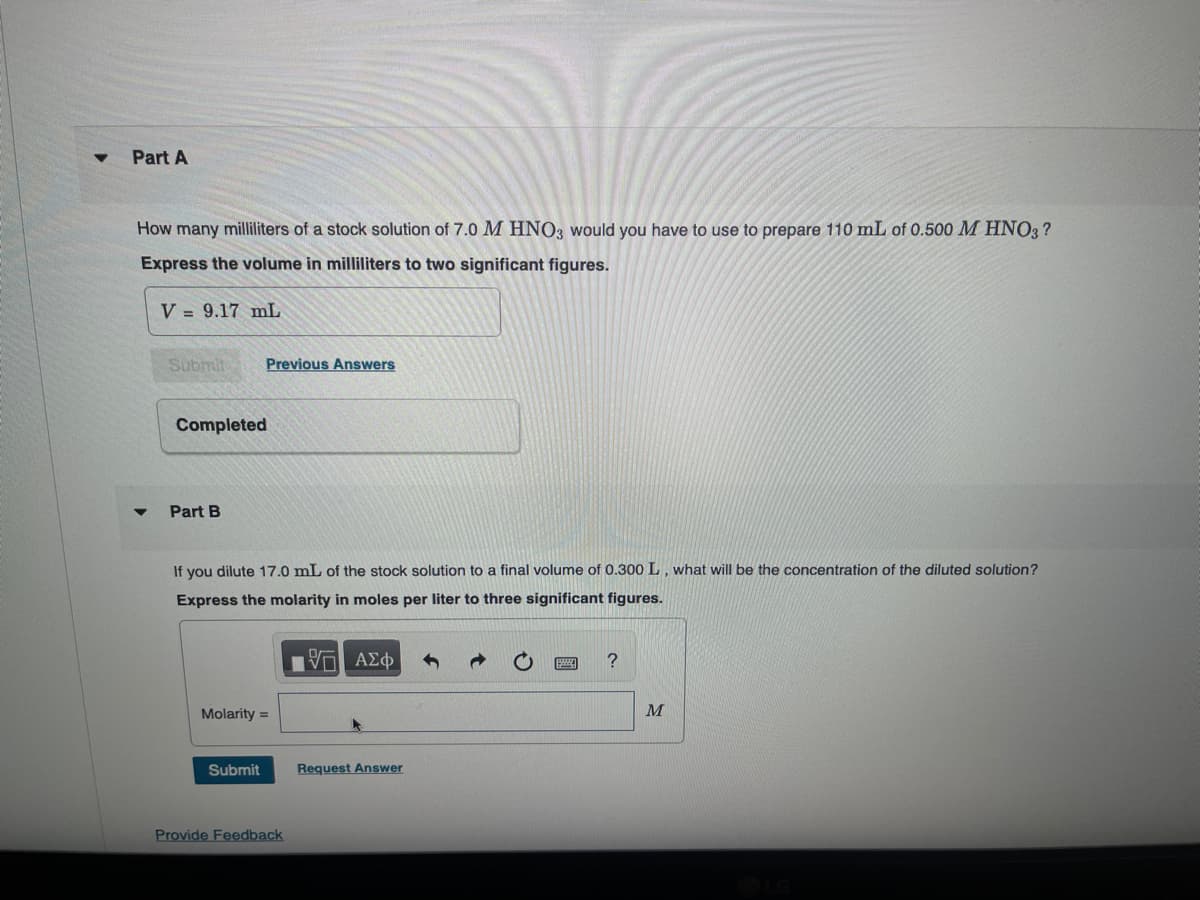
Transcribed Image Text:Part A
How many milliliters of a stock solution of 7.0 M HNO3 would you have to use to prepare 110 mL of 0.500 M HNO3?
Express the volume in milliliters to two significant figures.
V=9.17 mL
4
Submit
Previous Answers
Completed
Part B
If you dilute 17.0 mL of the stock solution to a final volume of 0.300 L, what will be the concentration of the diluted solution?
Express the molarity in moles per liter to three significant figures.
Molarity
=
V
Ο ΑΣΦ
Submit
Request Answer
Provide Feedback
M
LG
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
