4-tert-butylcyclohexanol 1,32 156.27 8.45.10 Which is the limiting reagent? Calculate the % yield of 4-tert-butylcyclohexanol (show working and note significant figures).
4-tert-butylcyclohexanol 1,32 156.27 8.45.10 Which is the limiting reagent? Calculate the % yield of 4-tert-butylcyclohexanol (show working and note significant figures).
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter5: Alkenes: Bonding, Nomenclature, And Properties
Section: Chapter Questions
Problem 5.31P
Related questions
Question
100%
Transcribed Image Text:2.
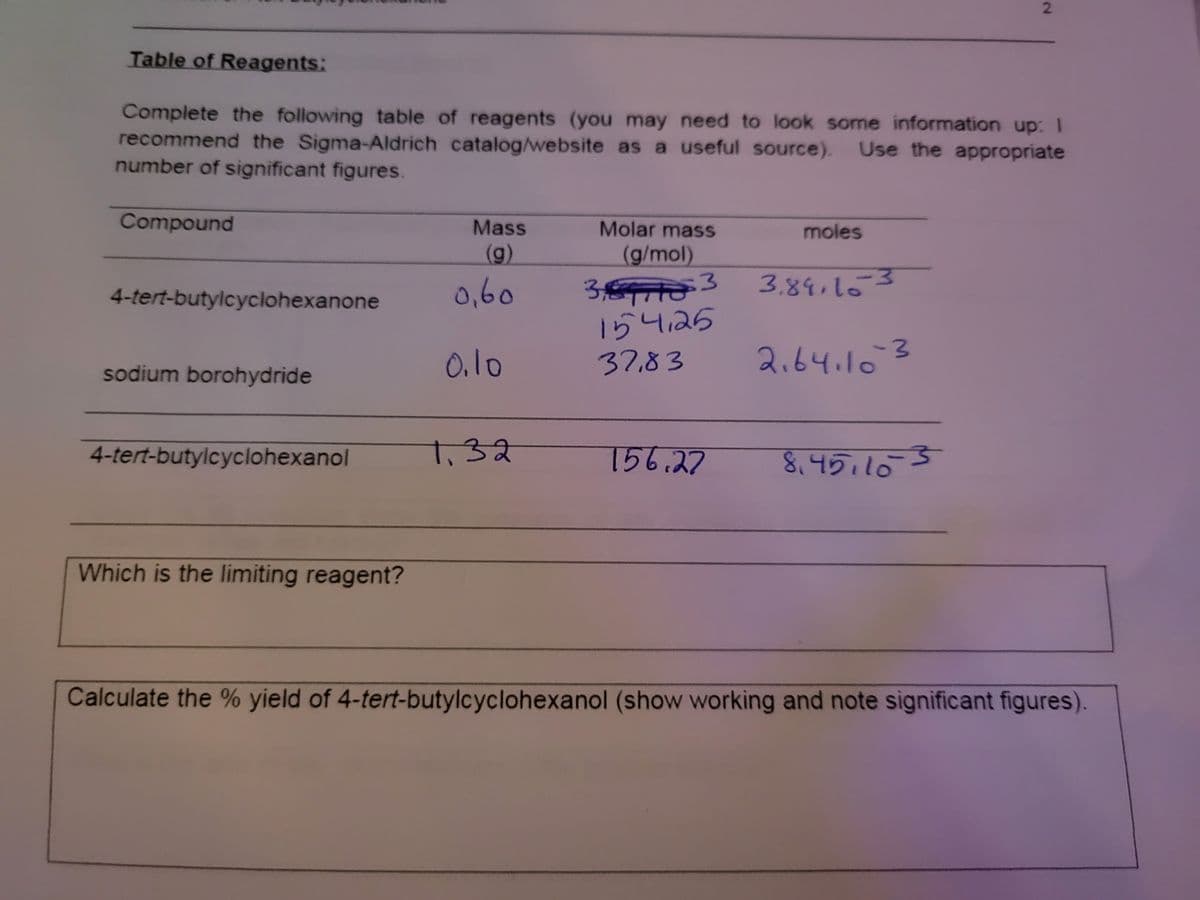
Table of Reagents:
Complete the following table of reagents (you may need to look some information up: 1
recommend the Sigma-Aldrich catalog/website as a useful source). Use the appropriate
number of significant figures.
Compound
Mass
Molar mass
moles
(g)
(g/mol)
3.
3,84,163
34
154,25
37,83
4-tert-butylcyclohexanone
0,60
0.10
2.64.163
sodium borohydride
4-tert-butylcyclohexanol
1.32
156.27
8.45.105
Which is the limiting reagent?
Calculate the % yield of 4-tert-butylcyclohexanol (show working and note significant figures).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning