4. At very high temperatures (like in early Universe) the gas molecules move with very high speeds (comparable to the speed of light), and therefore, one has to take relativis- tic effects into account. In this case the relation between energy and momentum is no longer the non-relativistic expression E = p²/2m, but it has to be replaced with the rel- ativistic relation E = |p|c, where c is the speed of light and |p| = /p; + p3+ p?. Repeat the same steps we carried out in the class to derive the partition function of a non- relativistic ideal gas and show that the partition function for a single molecule in the relativistic gas is given by V K³T³ . c3 8л (0.4)
4. At very high temperatures (like in early Universe) the gas molecules move with very high speeds (comparable to the speed of light), and therefore, one has to take relativis- tic effects into account. In this case the relation between energy and momentum is no longer the non-relativistic expression E = p²/2m, but it has to be replaced with the rel- ativistic relation E = |p|c, where c is the speed of light and |p| = /p; + p3+ p?. Repeat the same steps we carried out in the class to derive the partition function of a non- relativistic ideal gas and show that the partition function for a single molecule in the relativistic gas is given by V K³T³ . c3 8л (0.4)
Related questions
Question
Parts 1,2 and 3 have been answers in a previous so 3-6 is what I need to be solved.

Transcribed Image Text:4. At very high temperatures (like in early Universe) the gas molecules move with very
high speeds (comparable to the speed of light), and therefore, one has to take relativis-
tic effects into account. In this case the relation between energy and momentum is no
longer the non-relativistic expression E = p²/2m, but it has to be replaced with the rel-
ativistic relation E = |p|c, where c is the speed of light and |p| = /p; + p3+ p?. Repeat
the same steps we carried out in the class to derive the partition function of a non-
relativistic ideal gas and show that the partition function for a single molecule in the
relativistic gas is given by
V K³T³ .
c3
8л
(0.4)
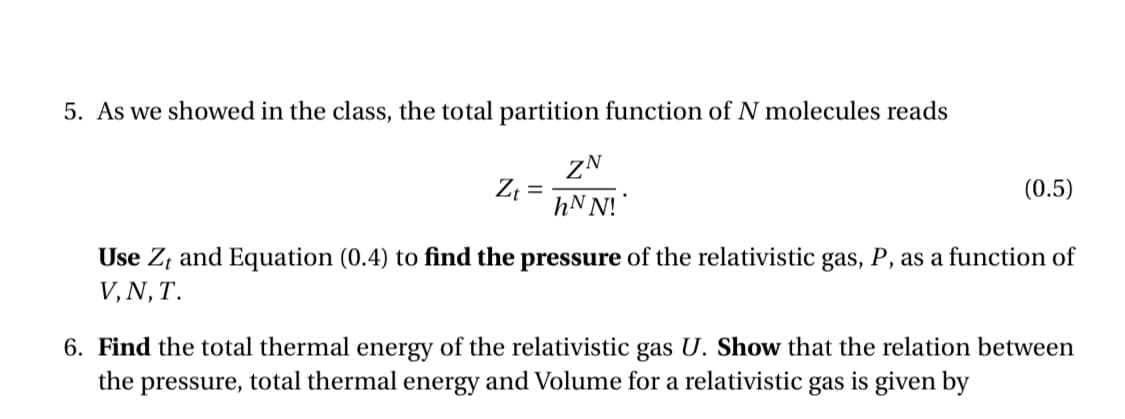
Transcribed Image Text:5. As we showed in the class, the total partition function of N molecules reads
ZN
hN N!
(0.5)
%D
Use Z, and Equation (0.4) to find the pressure of the relativistic gas, P, as a function of
V, N, T.
6. Find the total thermal energy of the relativistic gas U. Show that the relation between
the pressure, total thermal energy and Volume for a relativistic gas is given by
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 6 images
