4. The figure below shows a Px diagram for a binary system (component 1 and component 2) at 480 K. Black dots show data points from a series of constant mass expansion for four mixtures. Based on this diagram, answer the following: a) For a mixture of the two components to have a bubble point pressure of 6000 (kPa) at 480 K, what should be the overall composition of the mixture? b) Suppose you have 2.0 moles of component 1 and 2.0 moles of component 2 in the PVT cell at 6000 kPa and 480 K. The mixture shows two phases at equilibrium based on the diagram. What is the molar concentration of component 1 in the vapor phase? What is the molar concentration of component 1 in the liquid phase? c) What is the vapor mole fraction (Bv) for the two-phase systemin Part b? d) What is the K value for the component at 6000 kPa and 480 K? e) Is it possible for the binary mixture in part b to show a critical point at 480 K? Why or why not? 9000 8000 7000 Critical point
4. The figure below shows a Px diagram for a binary system (component 1 and component 2) at 480 K. Black dots show data points from a series of constant mass expansion for four mixtures. Based on this diagram, answer the following: a) For a mixture of the two components to have a bubble point pressure of 6000 (kPa) at 480 K, what should be the overall composition of the mixture? b) Suppose you have 2.0 moles of component 1 and 2.0 moles of component 2 in the PVT cell at 6000 kPa and 480 K. The mixture shows two phases at equilibrium based on the diagram. What is the molar concentration of component 1 in the vapor phase? What is the molar concentration of component 1 in the liquid phase? c) What is the vapor mole fraction (Bv) for the two-phase systemin Part b? d) What is the K value for the component at 6000 kPa and 480 K? e) Is it possible for the binary mixture in part b to show a critical point at 480 K? Why or why not? 9000 8000 7000 Critical point
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
Part c
Going to submit this question a few times for each part
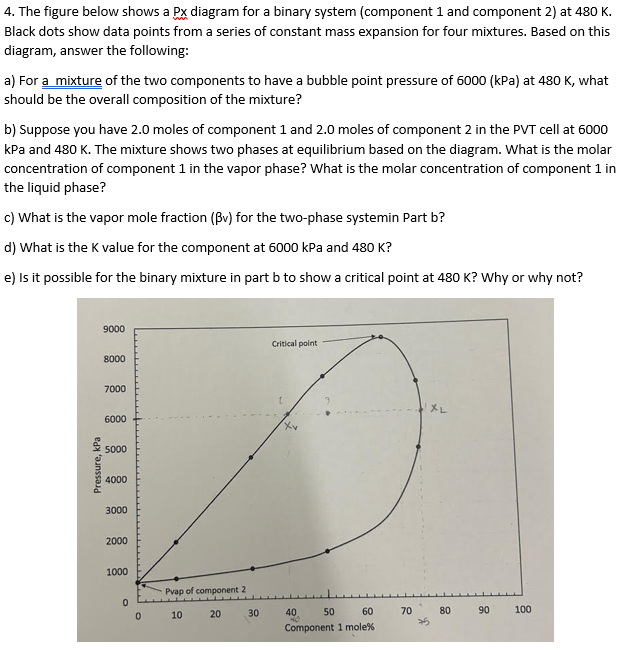
Transcribed Image Text:4. The figure below shows a Px diagram for a binary system (component 1 and component 2) at 480 K.
Black dots show data points from a series of constant mass expansion for four mixtures. Based on this
diagram, answer the following:
a) For a mixture of the two components to have a bubble point pressure of 6000 (kPa) at 480 K, what
should be the overall composition of the mixture?
b) Suppose you have 2.0 moles of component 1 and 2.0 moles of component 2 in the PVT cell at 6000
kPa and 480 K. The mixture shows two phases at equilibrium based on the diagram. What is the molar
concentration of component 1 in the vapor phase? What is the molar concentration of component 1 in
the liquid phase?
c) What is the vapor mole fraction (Bv) for the two-phase systemin Part b?
d) What is the K value for the component at 6000 kPa and 480 K?
e) Is it possible for the binary mixture in part b to show a critical point at 480 K? Why or why not?
Pressure, kPa
9000
8000
7000
6000
5000
4000
3000
2000
1000
0
0
Pvap of component 2
10
20
30
Critical point
40
50
60
Component 1 mole%
70
XL
80
90
100
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The