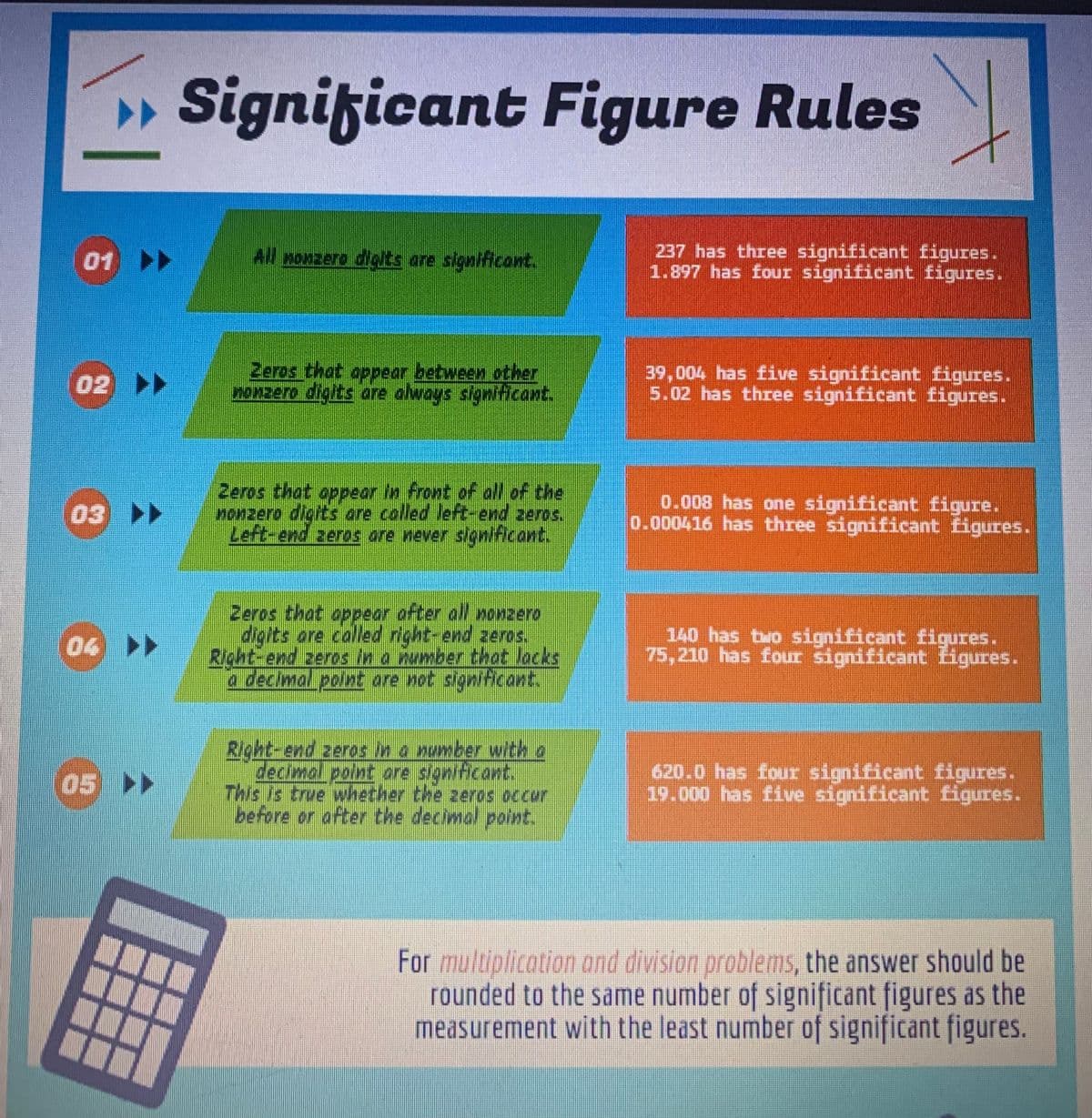
4.35 L He/1 x 1 mole He/22.4 L He. When we solve this problem we get 0.19419642. Apply the significant figure rules to the final answer. Then explain why the final answer makes sense using the example answer attached to help.
4.35 L He/1 x 1 mole He/22.4 L He. When we solve this problem we get 0.19419642. Apply the significant figure rules to the final answer. Then explain why the final answer makes sense using the example answer attached to help.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter2: Measurements And Calculations
Section: Chapter Questions
Problem 17ALQ: Complete the following and explain each in your own words: leading zeros are...
Related questions
Question
100%
4.35 L He/1 x 1 mole He/22.4 L He.
When we solve this problem we get 0.19419642. Apply the significant figure rules to the final answer.
Then explain why the final answer makes sense using the example answer attached to help.
Transcribed Image Text:01 ▶▶
02
03 >>
04 >>
05 >>
Significant Figure Rules
Rules
All nonzero digits are significant.
Zeros that appear between other
nonzero digits are always significant.
Zeros that appear in front of all of the
nonzero digits are called left-end zeros.
Left-end zeros are never significant.
Zeros that appear after all nonzero
digits are called right-end zeros.
Right-end zeros in a number that lacks
a decimal point are not significant.
Right-end zeros in a number with a
decimal point are significant.
This is true whether the zeros occur
before or after the decimal point.
237 has three significant figures.
1.897 has four significant figures.
39,004 has five significant figures.
5.02 has three significant figures.
0.008 has one significant figure.
0.000416 has three significant figures.
140 has two significant figures.
75,210 has four significant Ligures.
620.0 has four significant figures.
19.000 has five significant figures.
For multiplication and division problems, the answer should be
rounded to the same number of significant figures as the
measurement with the least number of significant figures.
Transcribed Image Text:Step 2: Calculate.
24 hr
1 d
1dx
X
60 min
1 hr
X
60 s
1 min
86, 400 s
%D
Applying the first conversion factor, the "d"
unit cancels and 1 × 24 = 24. Applying the
second conversion factor, the "hr" unit
cancels and 24 × 60 = 1440. Applying the
third conversion factor, the "min" unit cancels
and 1440 × 60 = 86, 400. The unit that remains
is "s" for seconds.
Step 3: Think about your result.
Seconds is a much smaller unit of time than
days, so it makes sense that there are a very
large number of seconds in one day.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning