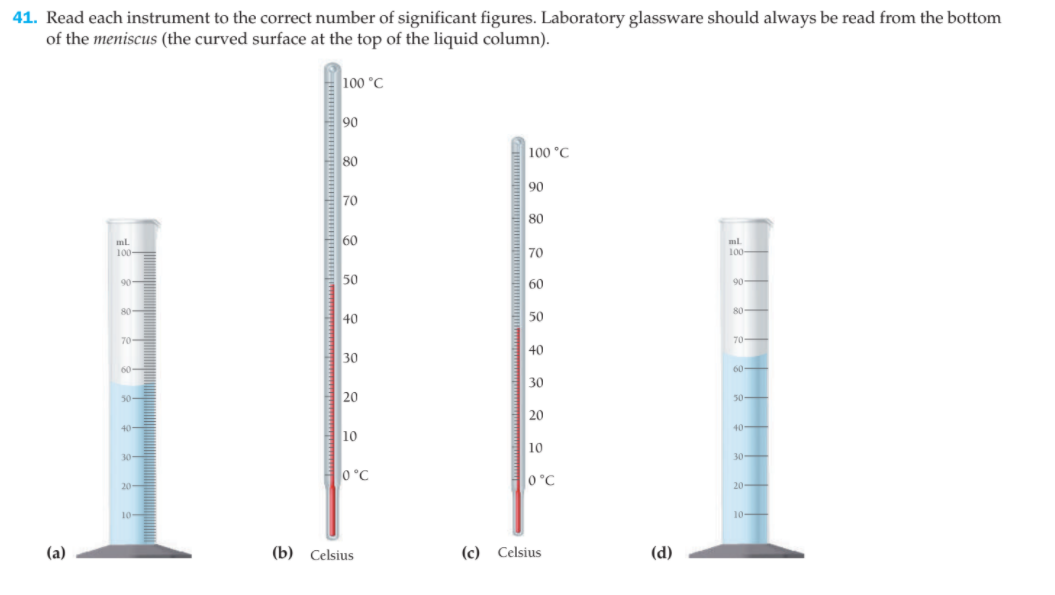
41. Read each instrument to the correct number of significant figures. Laboratory glassware should always be read from the bottom of the meniscus (the curved surface at the top of the liquid column). 100 °C 90 100 °C 80 90 70 80 60 ml. 100- ml 70 100 90 50 60 90- 80 80 40 50 70 - 70 40 30 60 60 30 20 30 50- 20 40 - 40 10 10 30- 30 0°C 0 °C 20 - 20 - 10 10- (a) (b) Celsius (c) Celsius (d)
41. Read each instrument to the correct number of significant figures. Laboratory glassware should always be read from the bottom of the meniscus (the curved surface at the top of the liquid column). 100 °C 90 100 °C 80 90 70 80 60 ml. 100- ml 70 100 90 50 60 90- 80 80 40 50 70 - 70 40 30 60 60 30 20 30 50- 20 40 - 40 10 10 30- 30 0°C 0 °C 20 - 20 - 10 10- (a) (b) Celsius (c) Celsius (d)
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter1: Basic Concepts Of Chemistry
Section: Chapter Questions
Problem 18PS: Which part of the description of a compound or element refers to its physical properties and which...
Related questions
Question
Transcribed Image Text:41. Read each instrument to the correct number of significant figures. Laboratory glassware should always be read from the bottom
of the meniscus (the curved surface at the top of the liquid column).
100 °C
90
100 °C
80
90
70
80
60
ml.
100-
ml
70
100
90
50
60
90-
80
80
40
50
70
-
70
40
30
60
60
30
20
30
50-
20
40
-
40
10
10
30-
30
0°C
0 °C
20
-
20
-
10
10-
(a)
(b) Celsius
(c) Celsius
(d)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
