42. Suppose the treatment uses palladium-103, which has a half-life of 16.99 days. a) Find the decay rate, k, of palladium-103. b) How much energy (measured in rems) is transmitted in the first month if the initial rate of transmission is 10 rems per year? c) What is the total amount of energy that the implant will transmit to the body?
42. Suppose the treatment uses palladium-103, which has a half-life of 16.99 days. a) Find the decay rate, k, of palladium-103. b) How much energy (measured in rems) is transmitted in the first month if the initial rate of transmission is 10 rems per year? c) What is the total amount of energy that the implant will transmit to the body?
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter14: Nuclear Chemistry
Section: Chapter Questions
Problem 14.99PAE
Related questions
Question
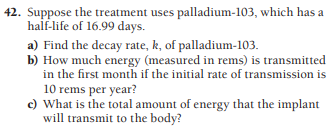
Transcribed Image Text:42. Suppose the treatment uses palladium-103, which has a
half-life of 16.99 days.
a) Find the decay rate, k, of palladium-103.
b) How much energy (measured in rems) is transmitted
in the first month if the initial rate of transmission is
10 rems per year?
c) What is the total amount of energy that the implant
will transmit to the body?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning
