49. This is the principle that best describes the colligative property represented by the illustration. * a. The addition of a volatile solute lowers the vapor pressure of a solution. O b. The addition of a volatile solvent lowers the vapor pressure of the solution. O c. The addition of a non-volatile solute lowers the vapor pressure of a given solution O d. The addition of a non-volatile solvent lowers the vapor pressure of a given solution 50. This is to establish an equilibrium within the solution of salt water. "
49. This is the principle that best describes the colligative property represented by the illustration. * a. The addition of a volatile solute lowers the vapor pressure of a solution. O b. The addition of a volatile solvent lowers the vapor pressure of the solution. O c. The addition of a non-volatile solute lowers the vapor pressure of a given solution O d. The addition of a non-volatile solvent lowers the vapor pressure of a given solution 50. This is to establish an equilibrium within the solution of salt water. "
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter10: Properties Of Solutions
Section: Chapter Questions
Problem 54E: At a certain temperature, the vapor pressure of pure benzene (C6H6) is 0.930 atm. A solution was...
Related questions
Question
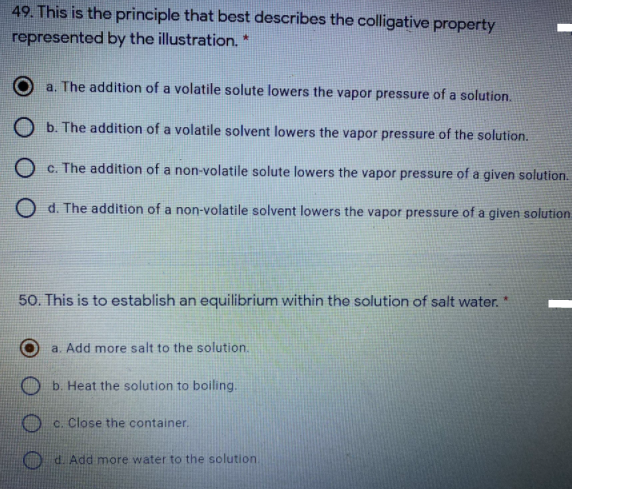
Transcribed Image Text:49. This is the principle that best describes the colligative property
represented by the illustration.
a. The addition of a volatile solute lowers the vapor pressure of a solution.
O b. The addition of a volatile solvent lowers the vapor pressure of the solution.
O c. The addition of a non-volatile solute lowers the vapor pressure of a given solution.
d. The addition of a non-volatile solvent lowers the vapor pressure of a given solution
50. This is to establish an equilibrium within the solution of salt water."
a. Add more salt to the solution.
b. Heat the solution to boiling.
O c. Close the container.
d. Add more water to the solution
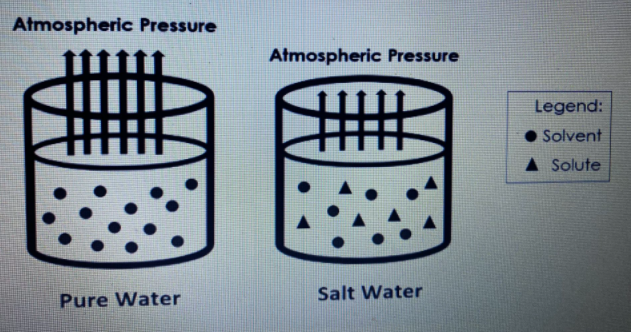
Transcribed Image Text:Atmospheric Pressure
Atmospheric Pressure
Legend:
• Solvent
A Solute
Salt Water
Pure Water
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT