5. Circle all of the following solutions that are buffered solutions. Justify your answer. (Ka (HC,H,O,) = 1.8 x 105; K,(NH,)= 1.8 x 105) a. 0.333 mol HC₂H₂O₂ and 0.111 mol KC₂H₂O₂ in 1.00 L solution b. 0.175 mol NaOH and 0.250 mol NH4Cl in 1.00 L solution c. 0.300 mol NaC₂H₂O₂ and 0.225 mol HCl in 1.00 L solution d. 0.200 mol HC₂H₂O, and 0.200 mol KOH in
5. Circle all of the following solutions that are buffered solutions. Justify your answer. (Ka (HC,H,O,) = 1.8 x 105; K,(NH,)= 1.8 x 105) a. 0.333 mol HC₂H₂O₂ and 0.111 mol KC₂H₂O₂ in 1.00 L solution b. 0.175 mol NaOH and 0.250 mol NH4Cl in 1.00 L solution c. 0.300 mol NaC₂H₂O₂ and 0.225 mol HCl in 1.00 L solution d. 0.200 mol HC₂H₂O, and 0.200 mol KOH in
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter16: Principles Of Chemical Reactivity: The Chemistry Of Acids And Bases
Section16.10: The Lewis Concept Of Acids And Bases
Problem 2.5ACP: To measure the relative strengths of bases stronger than OH, it is necessary to choose a solvent...
Related questions
Question
Solutions to question 5 please
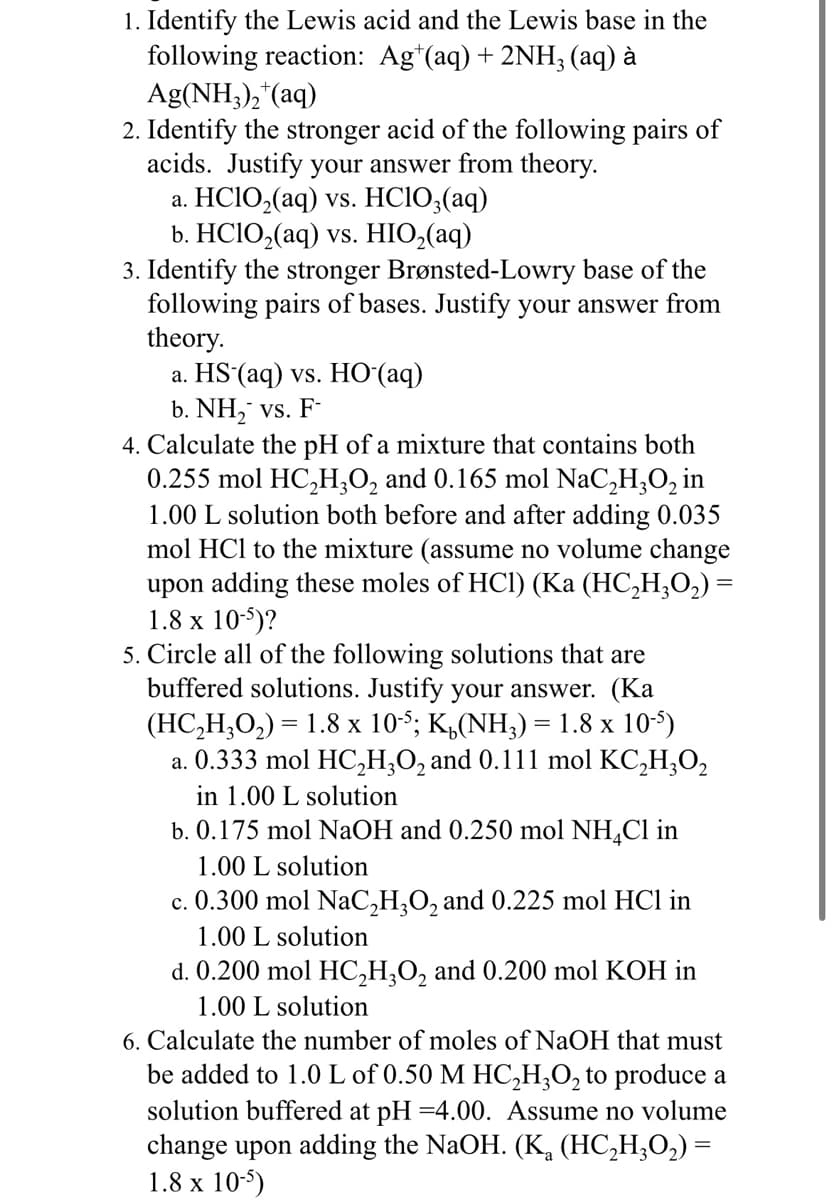
Transcribed Image Text:1. Identify the Lewis acid and the Lewis base in the
following reaction: Agt(aq) + 2NH₂ (aq) à
Ag(NH3)₂+(aq)
2. Identify the stronger acid of the following pairs of
acids. Justify your answer from theory.
a. HC1O,(aq) vs. HC1O,(aq)
b. HClO,(aq) vs. HIO,(aq)
3. Identify the stronger Brønsted-Lowry base of the
following pairs of bases. Justify your answer from
theory.
a. HS (aq) vs. HO (aq)
b. NH₂ vs. F-
4. Calculate the pH of a mixture that contains both
0.255 mol HC₂H3O₂ and 0.165 mol NaC₂H₂O₂ in
1.00 L solution both before and after adding 0.035
mol HCl to the mixture (assume no volume change
upon adding these moles of HCl) (Ka (HC₂H₂O₂) =
1.8 x 10-5)?
5. Circle all of the following solutions that are
buffered solutions. Justify your answer. (Ka
(HC,H,O,) = 1.8 x 10; K,(NH,)= 1.8 x 105)
a. 0.333 mol HC₂H₂O₂ and 0.111 mol KC₂H₂O₂
in 1.00 L solution
b. 0.175 mol NaOH and 0.250 mol NH₂Cl in
1.00 L solution
c. 0.300 mol NaC₂H₂O₂ and 0.225 mol HCl in
1.00 L solution
d. 0.200 mol HC₂H₂O2 and 0.200 mol KOH in
1.00 L solution
6. Calculate the number of moles of NaOH that must
be added to 1.0 L of 0.50 M HC₂H3O₂ to produce a
solution buffered at pH=4.00. Assume no volume
change upon adding the NaOH. (K₂ (HC₂H₂O₂) =
1.8 x 10-5)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 16 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning