5. Enthalpy of formation a. For which of these reactions at 25 °C does the enthalpy change represent a standard enthalpy of formation? For each that does not, what changes are needed to make it an equation whose AH is an enthalpy of formation? i. 2Na(s)+÷02(g)¬Na20(s) ii. 2K(l)+Cl½(g)→2KCI(s)
5. Enthalpy of formation a. For which of these reactions at 25 °C does the enthalpy change represent a standard enthalpy of formation? For each that does not, what changes are needed to make it an equation whose AH is an enthalpy of formation? i. 2Na(s)+÷02(g)¬Na20(s) ii. 2K(l)+Cl½(g)→2KCI(s)
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 5.99QE
Related questions
Question
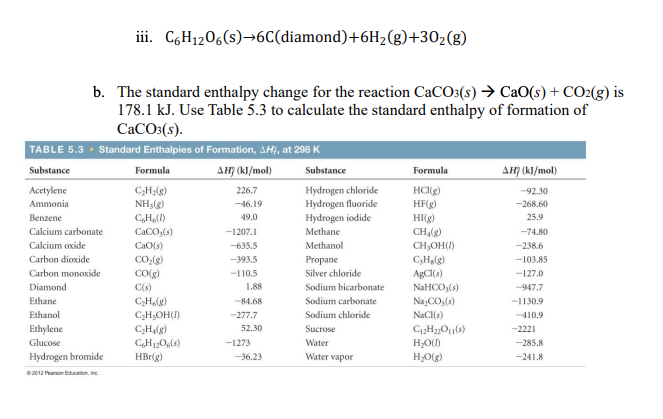
Transcribed Image Text:iii. C6H1206(s)→6C(diamond)+6H2(g)+302(g)
b. The standard enthalpy change for the reaction CaCO:(s) → CaO(s) + CO2(g) is
178.1 kJ. Use Table 5.3 to calculate the standard enthalpy of formation of
CaCO:(s).
TABLE 5.3 · Standard Enthalpies of Formation, AH, at 298 K
Substance
Formula
AH} (kl/mol)
Substance
Formula
AHF (kJ/mol)
Hydrogen chloride
Hydrogen fluoride
Hydrogen iodide
Acetylene
HC(g)
HF(g)
226.7
-92.30
Ammonia
NH3(g)
-46.19
-268.60
Benzene
CH(1)
49.0
HI(g)
25.9
Calcium carbonate
CaCO,(s)
-1207.1
Methane
CH,(g)
-74.80
Calcium oxide
CaO(s)
-635.5
Methanol
CH,OH()
-238.6
Carbon dioxide
CO:(g)
-393.5
Propane
C,Hlg)
-103.85
Carbon monoxide
COlg)
-110.5
Silver chloride
AgCI(s)
-127.0
Diamond
C(s)
1.88
Sodium bicarbonate
NAHCO,(s)
-947.7
Ethane
C,Holg)
C;H;OH()
-84.68
Sodium carbonate
Na,CO,(s)
-1130.9
Ethanol
-277.7
Sodium chloride
NaCl(s)
-410.9
Ethylene
Sucrose
CHlg)
CH120,(s)
52.30
-2221
Glucose
-1273
Water
H,O()
-285.8
Hydrogen bromide
HBr(g)
-36.23
Water vapor
H;O(g)
-241.8
2012 Pearon Bducation, Inc
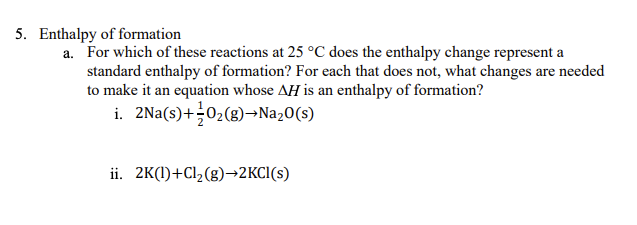
Transcribed Image Text:5. Enthalpy of formation
a. For which of these reactions at 25 °C does the enthalpy change represent a
standard enthalpy of formation? For each that does not, what changes are needed
to make it an equation whose AH is an enthalpy of formation?
i. 2Na(s)+02(g)→Na20(s)
ii. 2K(1)+Cl2(g)→2KCI(s)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning
