5. In the following table are listed various aqueous solutions. For one or more of the solutions assigned from the table, report the composition in the following ways:
5. In the following table are listed various aqueous solutions. For one or more of the solutions assigned from the table, report the composition in the following ways:
Chapter7: Solutions And Colloids
Section: Chapter Questions
Problem 7.42E
Related questions
Question
Need help answering sub questions a-d
please provide some short explanation
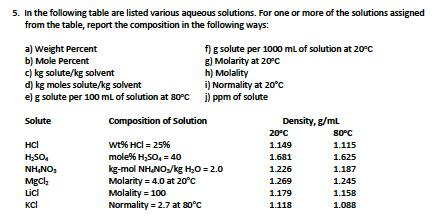
Transcribed Image Text:5. In the following table are listed various aqueous solutions. For one or more of the solutions assigned
from the table, report the composition in the following ways:
a) Weight Percent
b) Mole Percent
c) kg solute/kg solvent
d) kg moles solute/kg solvent
e) g solute per 100 ml of solution at 80°c ) ppm of solute
f) g solute per 1000 ml of solution at 20°c
g) Molarity at 20c
h) Molality
i) Normality at 20°c
Solute
Composition of Solution
Density, g/ml
20°C
80°C
wt% HCl = 25%
mole% H,SO, = 4o
kg-mol NHNO/kg H,O = 2.0
Molarity = 4.0 at 20°c
Molality = 100
Normality = 2.7 at 80°C
HCl
1.149
1.115
H,SO.
1.681
1.625
NH,NO,
MgCl,
1.226
1.187
1.269
1.245
Lici
1.179
1.158
KCl
1.118
1.088
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning