5. Measuring with a ruler using the metric system. The image below shows examples of blood that has been spun in a centrifuge to separate the red blood cells, white blood cells, and plasma. The percentage of blood which is composed of red blood cells is called the hematocrit (HCT). It is an important lab value because it indicates the blood's ability to carry oxygen to working tissues. Plasma: - Water, proteins, nutrients, hormones, etc. Buffy coat: - White blood cells, platelets Hematocrit: - Red blood cells Normal blood Total: RBC: In) bobbon om HCT: Normal Blood: 37% -47% hematocrit 42%-52% hematocrit with Solutiolations 1M 2.0 bas make euch o Anemia: Depressed hematocrit % HOMM Using your ruler measure the total amount of blood and the total amount of RBCs. Enter values in the table below. Use these measurements to calculate the %RBC (Hematocrit) of each of the samples above by taking the length of the RBC divided by the total length. Anemia Total: low RBC: 191Bw ins HCT: DER 1910 day Polycythemia: Elevated hematocrit % 202 203 Polycythemia Total: RBC: HCT:
5. Measuring with a ruler using the metric system. The image below shows examples of blood that has been spun in a centrifuge to separate the red blood cells, white blood cells, and plasma. The percentage of blood which is composed of red blood cells is called the hematocrit (HCT). It is an important lab value because it indicates the blood's ability to carry oxygen to working tissues. Plasma: - Water, proteins, nutrients, hormones, etc. Buffy coat: - White blood cells, platelets Hematocrit: - Red blood cells Normal blood Total: RBC: In) bobbon om HCT: Normal Blood: 37% -47% hematocrit 42%-52% hematocrit with Solutiolations 1M 2.0 bas make euch o Anemia: Depressed hematocrit % HOMM Using your ruler measure the total amount of blood and the total amount of RBCs. Enter values in the table below. Use these measurements to calculate the %RBC (Hematocrit) of each of the samples above by taking the length of the RBC divided by the total length. Anemia Total: low RBC: 191Bw ins HCT: DER 1910 day Polycythemia: Elevated hematocrit % 202 203 Polycythemia Total: RBC: HCT:
Chapter12: Water Requirements For Aquaculture
Section: Chapter Questions
Problem 27SA
Related questions
Question
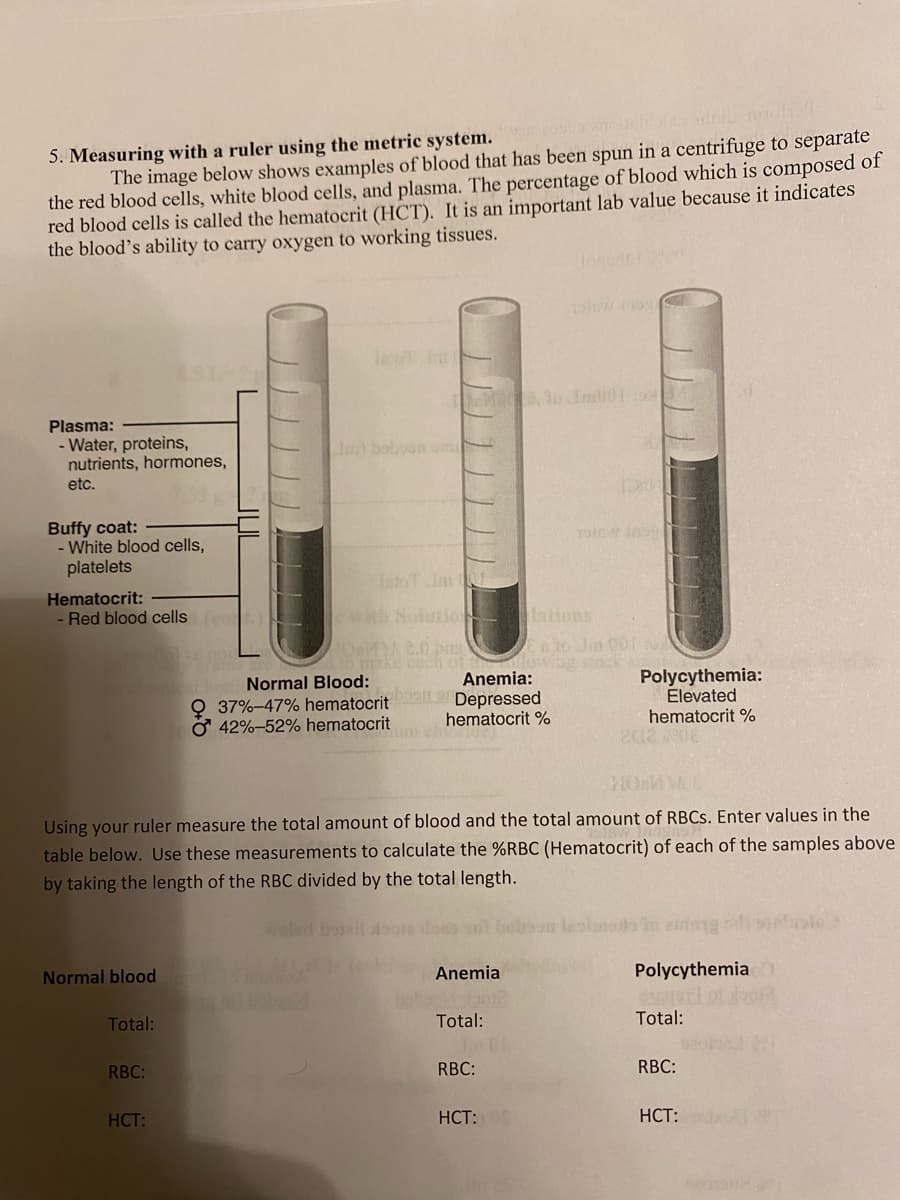
Transcribed Image Text:5. Measuring with a ruler using the metric system. scopun enolichi
The image below shows examples of blood that has been spun in a centrifuge to separate
the red blood cells, white blood cells, and plasma. The percentage of blood which is composed of
red blood cells is called the hematocrit (HCT). It is an important lab value because it indicates
the blood's ability to carry oxygen to working tissues.
Plasma:
- Water, proteins,
nutrients, hormones,
etc.
Buffy coat:
- White blood cells,
platelets
Hematocrit:
- Red blood cells (c
Normal blood
Total:
RBC:
HCT:
In) boboon om
Normal Blood:
37% -47% hematocrit
O 42%-52% hematocrit
100, 10 m01:00
with utiolations.
woled bassil
Anemia:
Depressed
hematocrit %
HOMM
Using your ruler measure the total amount of blood and the total amount of RBCs. Enter values in the
table below. Use these measurements to calculate the %RBC (Hematocrit) of each of the samples above
by taking the length of the RBC divided by the total length.
TolBW Joy
sech of a 30 Jm 007
lowing
Anemia
Total:
RBC:
DER
190EW inay
HCT:
Polycythemia:
Elevated
hematocrit %
202 202
Polycythemia
Total:
RBC:
HCT:

Transcribed Image Text:2.
3.
Perform all the calculations necessary for the volume of stock solution or water, in mL, needed to
make the dilute solutions listed below.
a.
b.
C.
Make: 200 mL of 30% Ethanol dilute solution
Stock
Volume needed (mL)
95% Ethanol
Reagent water
Make: 100mL of .09%NaCL
Stock
1% NaCl
Reagent water
30% SDS
3 M NaOH
Make: 100 mL of a 3% SDS and 0.5 MNaOH dilute solution as follows:
Stock
Volume needed (mL)
Reagent water
200 mL Total
1% Lactose
1% Dextrose
Volume needed (mL)
100 mL Total
Calculate the grams of chemical needed for each stock listed below.
Concentration of
Stock to Prepare
Volume of
Stock Needed
1% Sucrose
100 mL Total
10 mL
20 mL
25 mL
Amount of chemical
Needed (in grams)
Your instructor will have you make ONE of the above solutions-ask your instructor which solution to
make.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Recommended textbooks for you


Basic Clinical Lab Competencies for Respiratory C…
Nursing
ISBN:
9781285244662
Author:
White
Publisher:
Cengage



Basic Clinical Lab Competencies for Respiratory C…
Nursing
ISBN:
9781285244662
Author:
White
Publisher:
Cengage


Anatomy & Physiology
Biology
ISBN:
9781938168130
Author:
Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:
OpenStax College