5. Methanamide, CH,NO, is a liquid at 25°C. (a) The complete Lewis electron-dot diagram for methanamide is shown below. *O* H (i) In the molecule, angle x is not 180°. Estimate the observed angle. Justify your answer. (ii) In the molecule, angle y is not 90°. Explain why in terms of electron domains (VSEPR model). (b) Consider a molecule with the formula CH,O,. The structure of this molecule has a geometry around the carbon atom similar to the geometry around carbon in methanamide. In the box provided below, draw the complete Lewis electron-dot diagram for the molecule.
5. Methanamide, CH,NO, is a liquid at 25°C. (a) The complete Lewis electron-dot diagram for methanamide is shown below. *O* H (i) In the molecule, angle x is not 180°. Estimate the observed angle. Justify your answer. (ii) In the molecule, angle y is not 90°. Explain why in terms of electron domains (VSEPR model). (b) Consider a molecule with the formula CH,O,. The structure of this molecule has a geometry around the carbon atom similar to the geometry around carbon in methanamide. In the box provided below, draw the complete Lewis electron-dot diagram for the molecule.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter9: Bonding And Molecular Structure: Orbital Hybridization And Molecular Orbitals
Section9.2: Molecular Orbital Theory
Problem 9.6CYU: The cations O2+ and N2+ are formed when molecules of O2 and N2 are subjected to intense, high-energy...
Related questions
Question
thx!
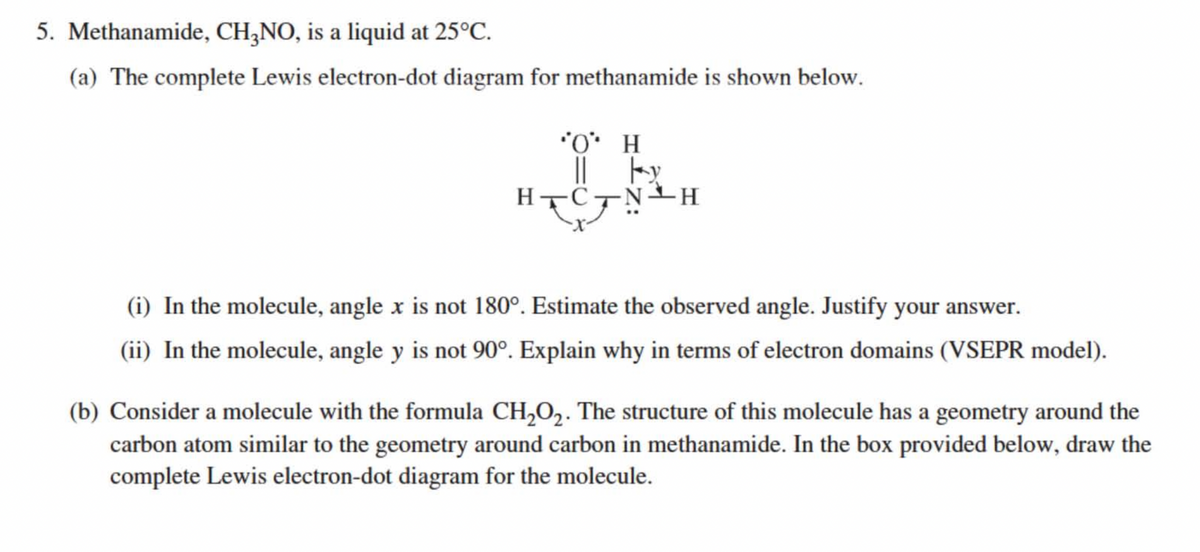
Transcribed Image Text:5. Methanamide, CH,NO, is a liquid at 25°C.
(a) The complete Lewis electron-dot diagram for methanamide is shown below.
*O* H
||
HTC
(i) In the molecule, angle x is not 180°. Estimate the observed angle. Justify your answer.
(ii) In the molecule, angle y is not 90°. Explain why in terms of electron domains (VSEPR model).
(b) Consider a molecule with the formula CH,O2. The structure of this molecule has a geometry around the
carbon atom similar to the geometry around carbon in methanamide. In the box provided below, draw the
complete Lewis electron-dot diagram for the molecule.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning