5. The tank in Example 3.8 has a leak that admits air at the rate. 0.0005lbm (Poutside - Piank) min atm m How long does it take the pump to reduce the pressure in the tank from 1 atm to 0.01 atm? What is the steady-state pressure in the tank? Vacuum Sys 38
5. The tank in Example 3.8 has a leak that admits air at the rate. 0.0005lbm (Poutside - Piank) min atm m How long does it take the pump to reduce the pressure in the tank from 1 atm to 0.01 atm? What is the steady-state pressure in the tank? Vacuum Sys 38
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
100%
Plz help I am so lost
I’m trying to solve for problem 5
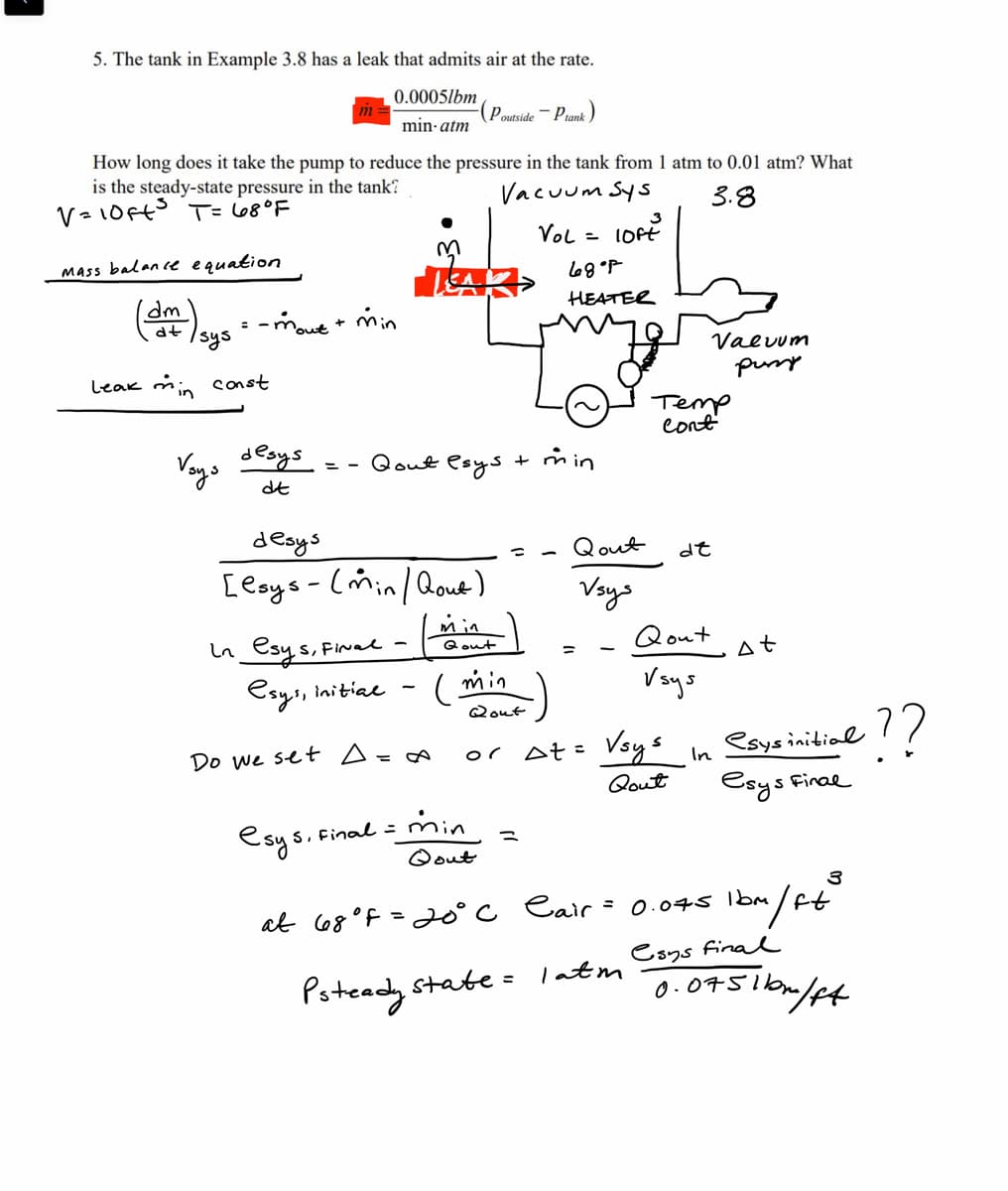
Transcribed Image Text:5. The tank in Example 3.8 has a leak that admits air at the rate.
0.0005lbm
min-atm
Mass balance equation
dm
dt
How long does it take the pump to reduce the pressure in the tank from 1 atm to 0.01 atm? What
is the steady-state pressure in the tank?
V = 10ft³ T = 68°F
Vacuum Sys
3.8
LOFE
Leak min
sys
= -mout + min
const
Voys
desys
dt
==
M
LEA
Qout esys
In esys, Final -
esys, initial
Do we set A=∞
Poutside
desys
[esys - (min/ Qout)
min
Qout
(min
esys, Final =
Qout
min
Qout
Plank
3
Vol =
+ min
at 68°F = 20°C
68°P
HEATER
or At=
Qout
Vsys
Cair
Psteady state = 1 atm
Temp
Cont
Vsys
Qout
de
Qout
Vsys
Valuum
pussy
In
At
esys initial
esys Final
0.075 1bm
m/ft
3
esys final
-??
0.0751bm/ft
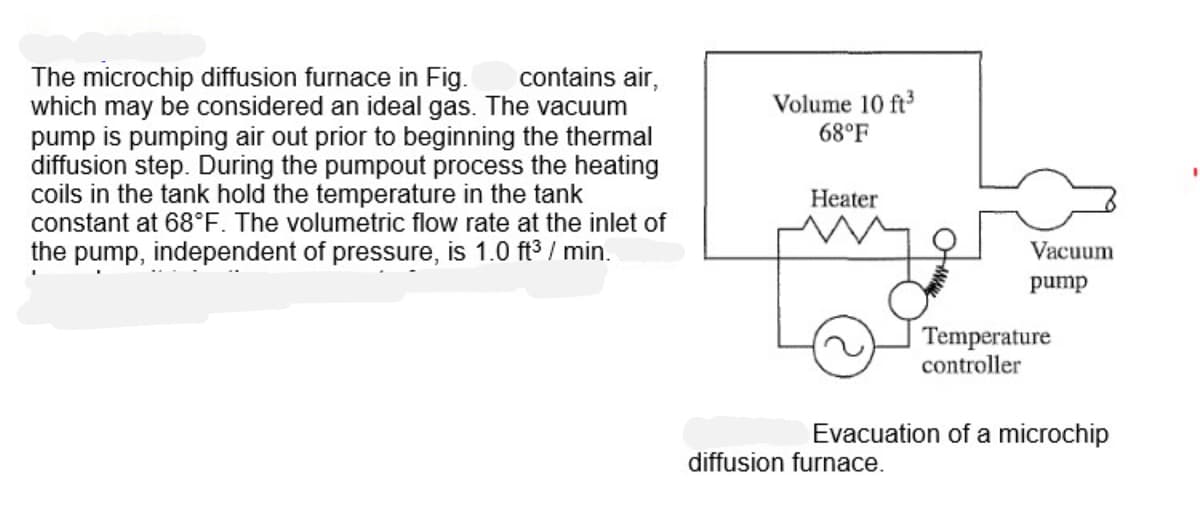
Transcribed Image Text:contains air,
The microchip diffusion furnace in Fig.
which may be considered an ideal gas. The vacuum
pump is pumping air out prior to beginning the thermal
diffusion step. During the pumpout process the heating
coils in the tank hold the temperature in the tank
constant at 68°F. The volumetric flow rate at the inlet of
the pump, independent of pressure, is 1.0 ft³/min.
Volume 10 ft³
68°F
Heater
Vacuum
pump
diffusion furnac
Temperature
controller
Evacuation of a microchip
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The