500 moles of a tetrahydrofuran (1)/o-xylene (2) containing 49 mol % tetrahydrofuran is distilled in an atmospheric batch still. A final tetrahydrofuran concentration of 2 mol % in the pot desired. Find the amount of distillate collected, the amount of waste left in the pot, and the average concentration of the distillate. Equilibrium data is given below for the binary system. Note: Use an interpolation interval of 0.01. Familibrium data for tatuabudnefunan (1) a rulone (2) binem. im
500 moles of a tetrahydrofuran (1)/o-xylene (2) containing 49 mol % tetrahydrofuran is distilled in an atmospheric batch still. A final tetrahydrofuran concentration of 2 mol % in the pot desired. Find the amount of distillate collected, the amount of waste left in the pot, and the average concentration of the distillate. Equilibrium data is given below for the binary system. Note: Use an interpolation interval of 0.01. Familibrium data for tatuabudnefunan (1) a rulone (2) binem. im
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
please help me with this one.
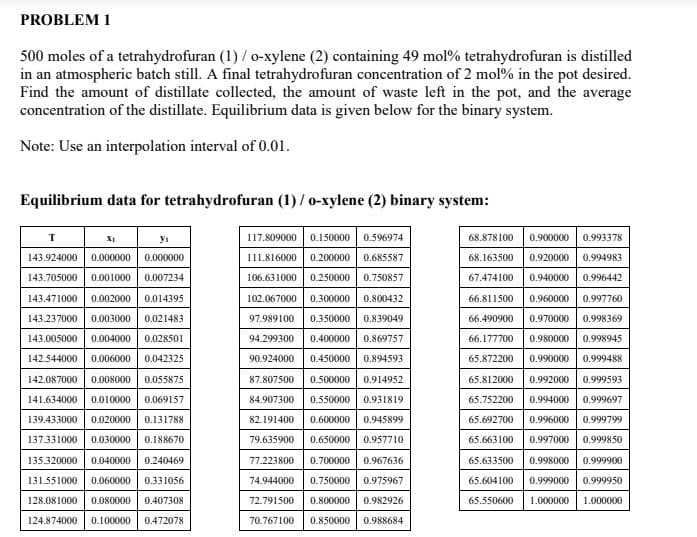
Transcribed Image Text:PROBLEM 1
500 moles of a tetrahydrofuran (1) / o-xylene (2) containing 49 mol% tetrahydrofuran is distilled
in an atmospheric batch still. A final tetrahydrofuran concentration of 2 mol % in the pot desired.
Find the amount of distillate collected, the amount of waste left in the pot, and the average
concentration of the distillate. Equilibrium data is given below for the binary system.
Note: Use an interpolation interval of 0.01.
Equilibrium data for tetrahydrofuran (1) / o-xylene (2) binary system:
117.809000 0.150000 0.596974
111.816000 0.200000 0.685587
106.631000 0.250000 0.750857
102.067000 0.300000 0.800432
97.989100 0.350000 0.839049
94.299300 0.400000 0.869757
90.924000 0.450000 0.894593
87.807500 0.500000 0.914952
84.907300 0.550000 0.931819
82.191400 0.600000 0.945899
79.635900 0.650000 0.957710
77.223800 0.700000 0.967636
74.944000 0.750000 0.975967
72.791500 0.800000 0.982926
70.767100 0.850000 0.988684
T
X1
yı
143.924000 0.000000 0.000000
143.705000 0.001000 0.007234
143.471000 0.002000 0.014395
143.237000 0.003000 0.021483
143.005000 0.004000 0.028501
142.544000 0.006000 0.042325
142.087000 0.008000 0.055875
141.634000 0.010000 0.069157
139.433000 0.020000 0.131788
137.331000 0.030000 0.188670
135.320000 0.040000 0.240469
131.551000 0.060000 0.331056
128.081000 0.080000 0.407308
124.874000 0.100000 0.472078
68.878100 0.900000 0.993378
68.163500 0.920000 0.994983
67.474100 0.940000 0.996442
66.811500 0.960000 0.997760
66.490900 0.970000 0.998369
66.177700 0.980000 0.998945
65.872200 0.990000 0.999488
65.812000 0.992000 0.999593
65.752200 0.994000 0.999697
65.692700 0.996000 0.999799
65.663100 0.997000 0.999850
65.633500 0.998000 0.999900
65.604100 0.999000 0.999950
65.550600 1.000000 1.000000
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The