50 mL of 0.15 M Cu2+ solution was prepared. 5 mL increments of 1.5 M NH4OH (onequivalent) were added in a calorimeter and the change in temperature were noted on each addition. Assuming the density of the solution is 1 g/mL and the specific heat of the solution is a constant 4.18 J/g C the following data were obtained: Trial 1-total volume 55 mL- T1 = 22C, T2 = 24.8C Trial 2-total Volume 60 mL, T1 = 22.3 C, T2 = 24.9C %3D Trial 3-total Volume 65 mL, T1 = 22.1C, T2 = 24.5C %3D Trial 4 - total Volume 70 mL, T1 = 21.9C, T2 = 24.1C Trial 5 - Total Volume 75 mL, T1 = T2 = 22.3C What is the Coordination Number of Cu2+ in Ammonia? What is the average dq for bond formation? What is the average Delta H (J/mol) of bond formation?
50 mL of 0.15 M Cu2+ solution was prepared. 5 mL increments of 1.5 M NH4OH (onequivalent) were added in a calorimeter and the change in temperature were noted on each addition. Assuming the density of the solution is 1 g/mL and the specific heat of the solution is a constant 4.18 J/g C the following data were obtained: Trial 1-total volume 55 mL- T1 = 22C, T2 = 24.8C Trial 2-total Volume 60 mL, T1 = 22.3 C, T2 = 24.9C %3D Trial 3-total Volume 65 mL, T1 = 22.1C, T2 = 24.5C %3D Trial 4 - total Volume 70 mL, T1 = 21.9C, T2 = 24.1C Trial 5 - Total Volume 75 mL, T1 = T2 = 22.3C What is the Coordination Number of Cu2+ in Ammonia? What is the average dq for bond formation? What is the average Delta H (J/mol) of bond formation?
Living By Chemistry: First Edition Textbook
1st Edition
ISBN:9781559539418
Author:Angelica Stacy
Publisher:Angelica Stacy
ChapterU5: Fire: Energy , Thermodynamics, And Oxidation-reduction
SectionU5.9: Counting Calories: Calorimetry Calculations
Problem 4E
Related questions
Question
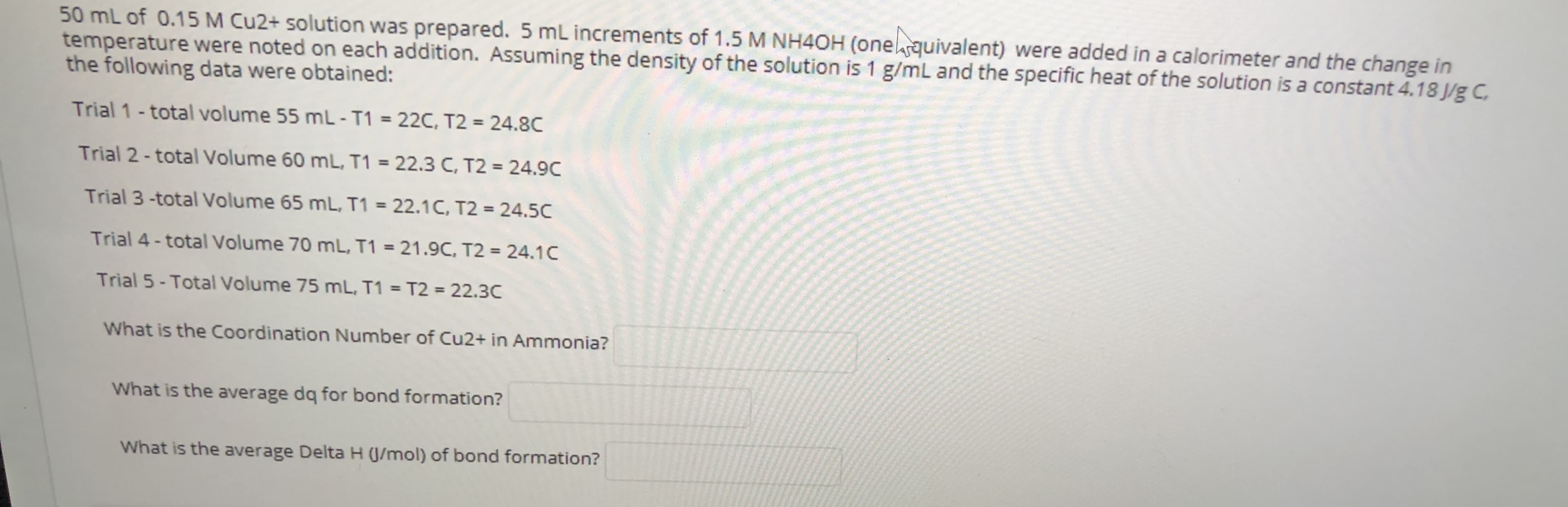
Transcribed Image Text:50 mL of 0.15 M Cu2+ solution was prepared. 5 mL increments of 1.5 M NH4OH (onequivalent) were added in a calorimeter and the change in
temperature were noted on each addition. Assuming the density of the solution is 1 g/mL and the specific heat of the solution is a constant 4.18 J/g C
the following data were obtained:
Trial 1-total volume 55 mL- T1 = 22C, T2 = 24.8C
Trial 2-total Volume 60 mL, T1 = 22.3 C, T2 = 24.9C
%3D
Trial 3-total Volume 65 mL, T1 = 22.1C, T2 = 24.5C
%3D
Trial 4 - total Volume 70 mL, T1 = 21.9C, T2 = 24.1C
Trial 5 - Total Volume 75 mL, T1 = T2 = 22.3C
What is the Coordination Number of Cu2+ in Ammonia?
What is the average dq for bond formation?
What is the average Delta H (J/mol) of bond formation?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 2 images

Recommended textbooks for you

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER