50.0 mL 2.00M HCI and 50.0 mL 2.00 M NaOH mixed: HCI(aq) + NaOH(aq) → H,0() + NaCI(aq) - Csoln = Cwater = 4.184 J/(g °C) dsoln = dwater = 1.00 g/mL - mgoln = (50 mL + 50 mL) = 100 mL = 100 g - ΔΤ, %3D %3D %3D %3D AT 13.8 °C (same mass water, twice the moles) soln Asoin = (100 g)(4.184 J/(g °C)(13.8 °C) = +5773.92 J - Moles HCI: (M*L) = (2.00 M)(0.050 L) = 0.100 moles - Moles NaOH = (2.00 M)(0.050 L) = 0.100 moles %3D %3D %3D %3D %3D 9soln = +5773.92 J/0.100 moles = +57,739.2 J J/mole AHn = -57.7 kJ/mole %3D rxn Pearson Education, Inc.
50.0 mL 2.00M HCI and 50.0 mL 2.00 M NaOH mixed: HCI(aq) + NaOH(aq) → H,0() + NaCI(aq) - Csoln = Cwater = 4.184 J/(g °C) dsoln = dwater = 1.00 g/mL - mgoln = (50 mL + 50 mL) = 100 mL = 100 g - ΔΤ, %3D %3D %3D %3D AT 13.8 °C (same mass water, twice the moles) soln Asoin = (100 g)(4.184 J/(g °C)(13.8 °C) = +5773.92 J - Moles HCI: (M*L) = (2.00 M)(0.050 L) = 0.100 moles - Moles NaOH = (2.00 M)(0.050 L) = 0.100 moles %3D %3D %3D %3D %3D 9soln = +5773.92 J/0.100 moles = +57,739.2 J J/mole AHn = -57.7 kJ/mole %3D rxn Pearson Education, Inc.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter6: Thermochemisty
Section: Chapter Questions
Problem 6.111QP: In a calorimetric experiment, 6.48 g of lithium hydroxide, LiOH, was dissolved in water. The...
Related questions
Question
100%
For the part circled, what if the molarity is different and one has a molarity of 3 and one has M=2. When we normalize, are we dividing by the limiting reactant?
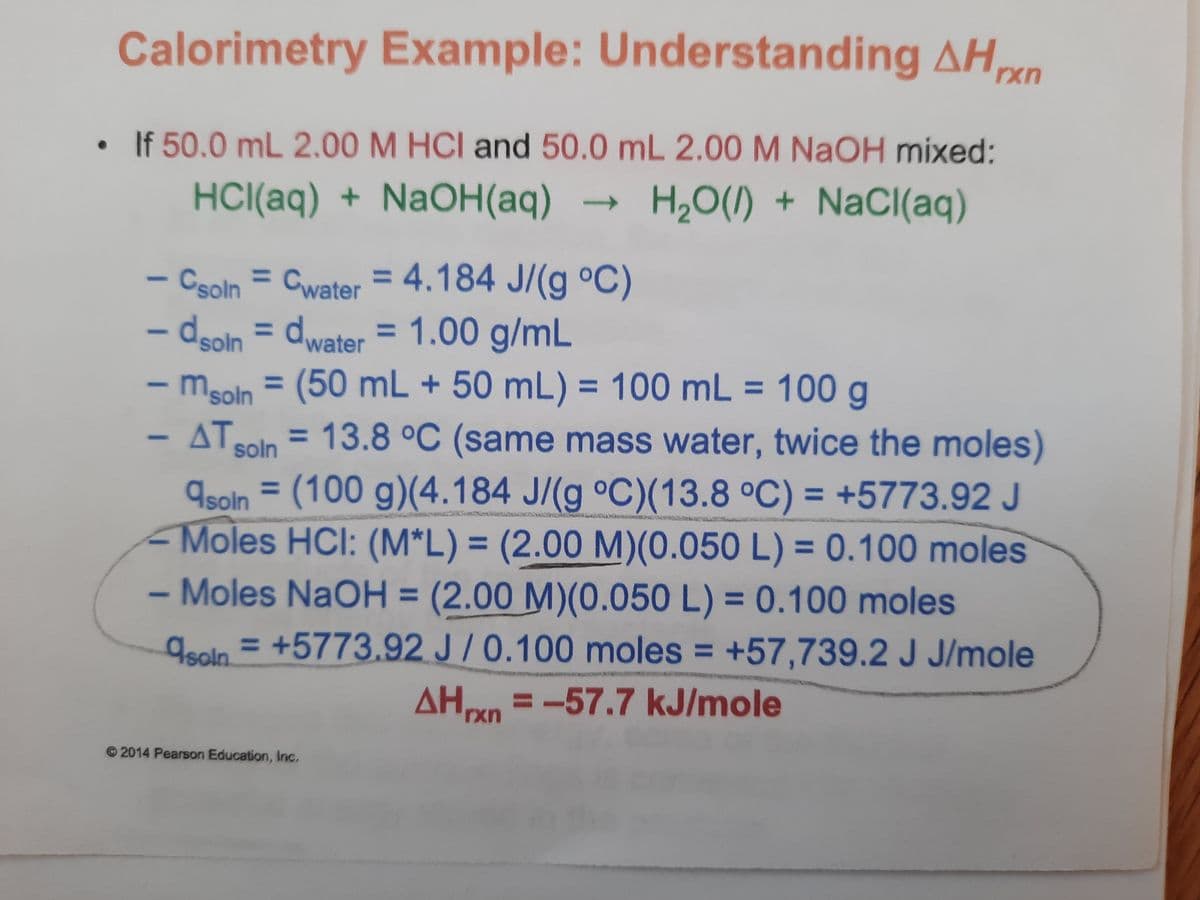
Transcribed Image Text:Calorimetry Example: Understanding AHn
If 50.0 mL 2.00 M HCI and 50.0 mL 2.00 M NaOH mixed:
HCI(aq) + NaOH(aq) →
H20(1) + NaCl(aq)
- Csoln = Cwater = 4.184 J/(g °C)
– dooln = dwater = 1.00 g/mL
- msoln = (50 mL + 50 mL) = 100 mL = 100 g
%3D
%3D
- ΔΤ,
ATSoln = 13.8 °C (same mass water, twice the moles)
%3D
9soln = (100 g)(4.184 J/(g °C)(13.8 °C) = +5773.92 J
Moles HCI: (M*L) = (2.00 M)(0.050 L) = 0.100 moles
Moles NaOH = (2.00 M)(0.050 L) = 0.100 moles
geoln = +5773.92 J /0.100 moles = +57,739.2 J J/mole
%3D
%3D
%3D
%3D
%3D
%3D
AHn =-57.7 kJ/mole
rxr
© 2014 Pearson Education, Inc.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning