6 The graphite anodes have to be replaced regularly. Why is this? 7 Why is it so much more cost-effective to recycle aluminium than to extract new aluminium?
6 The graphite anodes have to be replaced regularly. Why is this? 7 Why is it so much more cost-effective to recycle aluminium than to extract new aluminium?
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 119AE: The black silver sulfide discoloration of silverware can be removed by heating the silver article in...
Related questions
Question
number 6 and 7 only
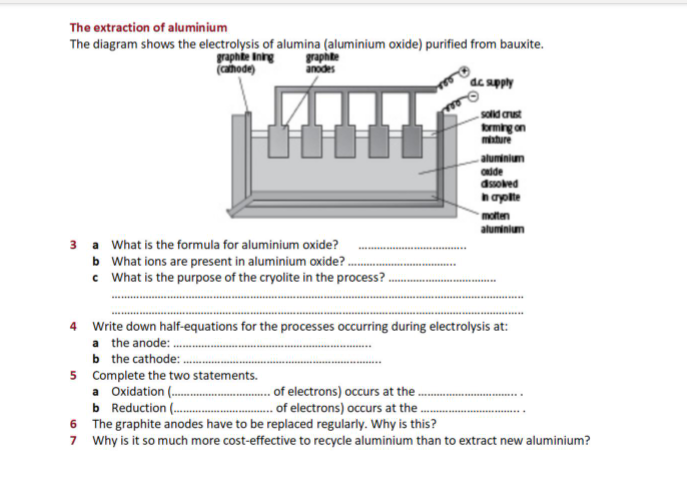
Transcribed Image Text:The extraction of aluminium
The diagram shows the electrolysis of alumina (aluminium oxide) purified from bauxite.
graphte
anodes
graphite Inng
(cahode)
dc apply
sold crust
krming on
minture
aluminium
oide
dsobed
hayolte
moten
aluminium
3 a What is the formula for aluminium oxide?
b What ions are present in aluminium oxide?
c What is the purpose of the cryolite in the process?
4 Write down half-equations for the processes occurring during electrolysis at:
a the anode: .
b the cathode:
5 Complete the two statements.
a Oxidation (.
b Reduction (.
6 The graphite anodes have to be replaced regularly. Why is this?
7 Why is it so much more cost-effective to recycle aluminium than to extract new aluminium?
- of electrons) occurs at the.
... of electrons) occurs at the .
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax