6. Cs-137 emits gamma photons with a principle energy of 662 keV while Tc-99m has a principle photon energy of 140 keV. Would you expect to have about the same exposure reading (mR/hr) of 90 mCi of Tc-99m at 1 meter as you would for 90 mCi of Cs-137 at 1 meter? Why or why not? (Hint-multiple detector characteristics may impact your answer.)
6. Cs-137 emits gamma photons with a principle energy of 662 keV while Tc-99m has a principle photon energy of 140 keV. Would you expect to have about the same exposure reading (mR/hr) of 90 mCi of Tc-99m at 1 meter as you would for 90 mCi of Cs-137 at 1 meter? Why or why not? (Hint-multiple detector characteristics may impact your answer.)
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter6: The Periodic Table And Atomic Structure
Section: Chapter Questions
Problem 6.21PAE: 6.21 The electron binding energy fur copper metal is 7.181019J . Find the longest wavelength of...
Related questions
Question
4
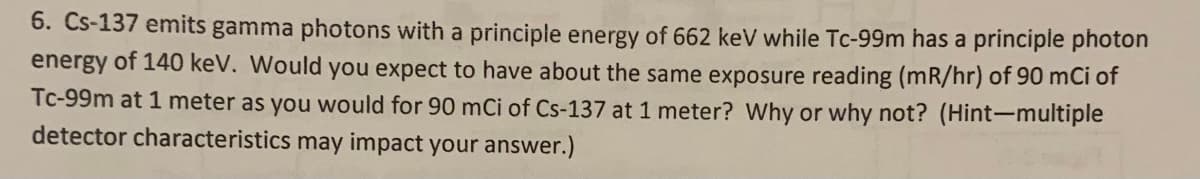
Transcribed Image Text:6. Cs-137 emits gamma photons with a principle energy of 662 keV while Tc-99m has a principle photon
energy of 140 keV. Would you expect to have about the same exposure reading (mR/hr) of 90 mCi of
Tc-99m at 1 meter as you would for 90 mCi of Cs-137 at 1 meter? Why or why not? (Hint-multiple
detector characteristics may impact your answer.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning