6. Imagine the experiment below where you placed a dialysis tube (cell) containing 10% NaCl Solution A Dialysis Tube Solution B (solution B) into a beaker of distilled water (solution A). Using what you know about diffusion/osmosis, indicate if the water is going to move into or out of the dialysis 'cell’.
6. Imagine the experiment below where you placed a dialysis tube (cell) containing 10% NaCl Solution A Dialysis Tube Solution B (solution B) into a beaker of distilled water (solution A). Using what you know about diffusion/osmosis, indicate if the water is going to move into or out of the dialysis 'cell’.
Chapter17: Respiratory System
Section: Chapter Questions
Problem 2RQ
Related questions
Question
100%
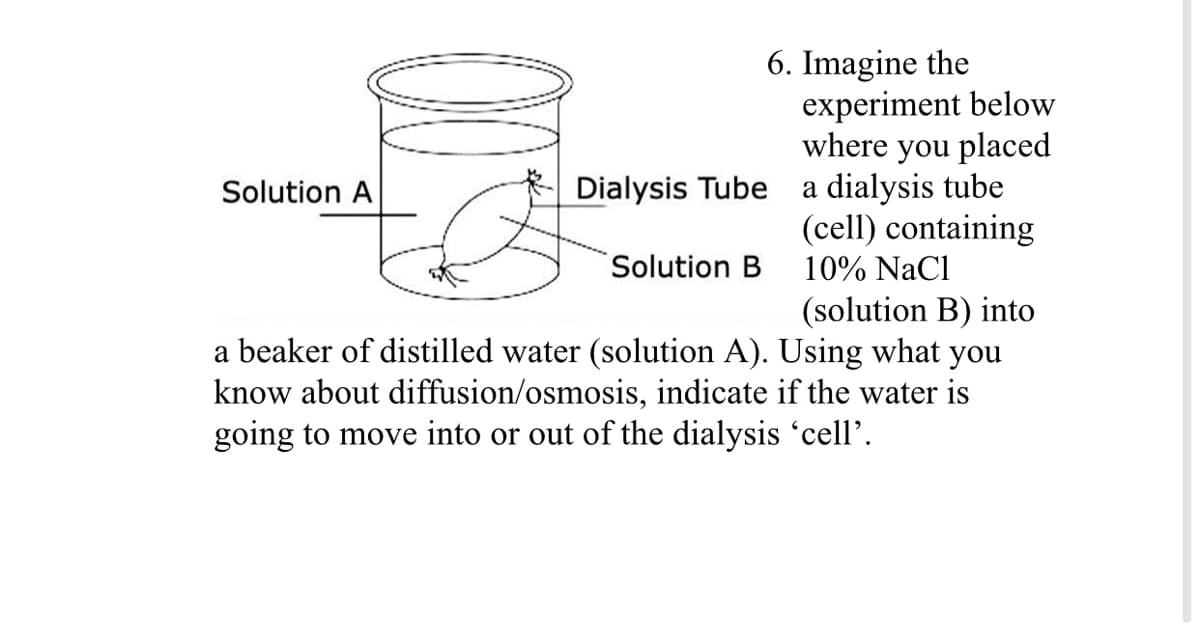
Transcribed Image Text:6. Imagine the
experiment below
where you placed
a dialysis tube
(cell) containing
Solution A
Dialysis Tube
Solution B
10% NaCl
(solution B) into
a beaker of distilled water (solution A). Using what you
know about diffusion/osmosis, indicate if the water is
going to move into or out of the dialysis 'cell'.

Transcribed Image Text:9. Using the images of blood from 7.8 in the lab manual, what
is the ideal tonicity of sheep's blood? Explain.
10. Using the image in 7.9 of the Elodea cell tissue in the lab
manual, why is a hypertonic environment dangerous to
plant cells? Explain.
11. Review the following experiment and answer the
questions.
You set up an experiment where you place a sealed
(clamped) dialysis tube cell in a beaker of water. The
solution in the dialysis tube cell is 25%NaCl solution and
the solution in the beaker is 1%NaCl solution. The solvent
for both is water.
Before you place the tube in the cell, after it has been filled
and clamped, you find the mass of the cell. You also find
the mass of the cell after it has been placed in the beaker
solution for 30 minutes. The masses are in the table below.
Mass in grams of dialysis tube cell
Initial Mass
25g
Final Mass after 30 minutes 28.7g
Using this information, explain the difference in mass
before and after submerging the cell in the beaker solution.
Explain.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Includes step-by-step video
Trending now
This is a popular solution!
Learn your way
Includes step-by-step video
Step by step
Solved in 2 steps

Recommended textbooks for you


Anatomy & Physiology
Biology
ISBN:
9781938168130
Author:
Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:
OpenStax College

Cardiopulmonary Anatomy & Physiology
Biology
ISBN:
9781337794909
Author:
Des Jardins, Terry.
Publisher:
Cengage Learning,


Anatomy & Physiology
Biology
ISBN:
9781938168130
Author:
Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:
OpenStax College

Cardiopulmonary Anatomy & Physiology
Biology
ISBN:
9781337794909
Author:
Des Jardins, Terry.
Publisher:
Cengage Learning,