6. Predict the products and write balanced net ionic equations for the following reactions. (g) SnClz is added to KMNO4 solution (acidic) forming Mn²".
6. Predict the products and write balanced net ionic equations for the following reactions. (g) SnClz is added to KMNO4 solution (acidic) forming Mn²".
Chapter23: Voltammetry
Section: Chapter Questions
Problem 23.9QAP
Related questions
Question
Using the Atoms, Oxygen, Hydrogen, and Electron (AOHE) method please help me balance the oxidation/reduction reactions I provided an example below on how to solve it using this method thank you so much!
Directions: Predict the products and write balanced net ionic equations for the folllowing reactions

Transcribed Image Text:6. Predict the products and write balanced net ionic equations for the following reactions.
(s) SnCl; is added to KMNO4 solution (acidic) forming Mn".
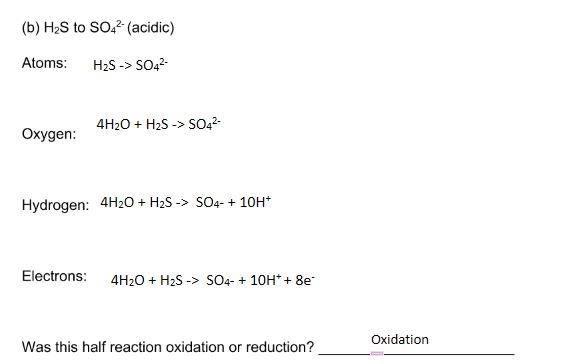
Transcribed Image Text:(b) H2S to SO,2 (acidic)
Atoms:
H2S -> SO,2-
4H20 + H2S -> SO42-
Охудen:
Hydrogen: 4H20 + H2S -> SO4- + 10H*
Electrons:
4H20 + H2S -> SO4- + 10H* + 8e
Oxidation
Was this half reaction oxidation or reduction?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning