6. The precise mass of a compound was determined by high resolution mass spec to be 84.0575. Using the values in the precise atomic mass table below determine which molecular formula best agrees with this mass- C6H12, C5H,O or C4H&N2? Table 13.3 The Exact Masses of Some Common Isotopes Mass Isotope Mass Isotope 31.9721 amu 1.007825 amu 32S 34.9689 amu 12C 12.00000 amu 35CI 78.9183 amu 79B. 14.0031 amu 14N 15.9949 amu 160
6. The precise mass of a compound was determined by high resolution mass spec to be 84.0575. Using the values in the precise atomic mass table below determine which molecular formula best agrees with this mass- C6H12, C5H,O or C4H&N2? Table 13.3 The Exact Masses of Some Common Isotopes Mass Isotope Mass Isotope 31.9721 amu 1.007825 amu 32S 34.9689 amu 12C 12.00000 amu 35CI 78.9183 amu 79B. 14.0031 amu 14N 15.9949 amu 160
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter2: Atoms, Molecules, And Ions
Section: Chapter Questions
Problem 2.11QE: A mass spectrometer determines isotopic masses to eight or nine significant digits. What limits the...
Related questions
Question
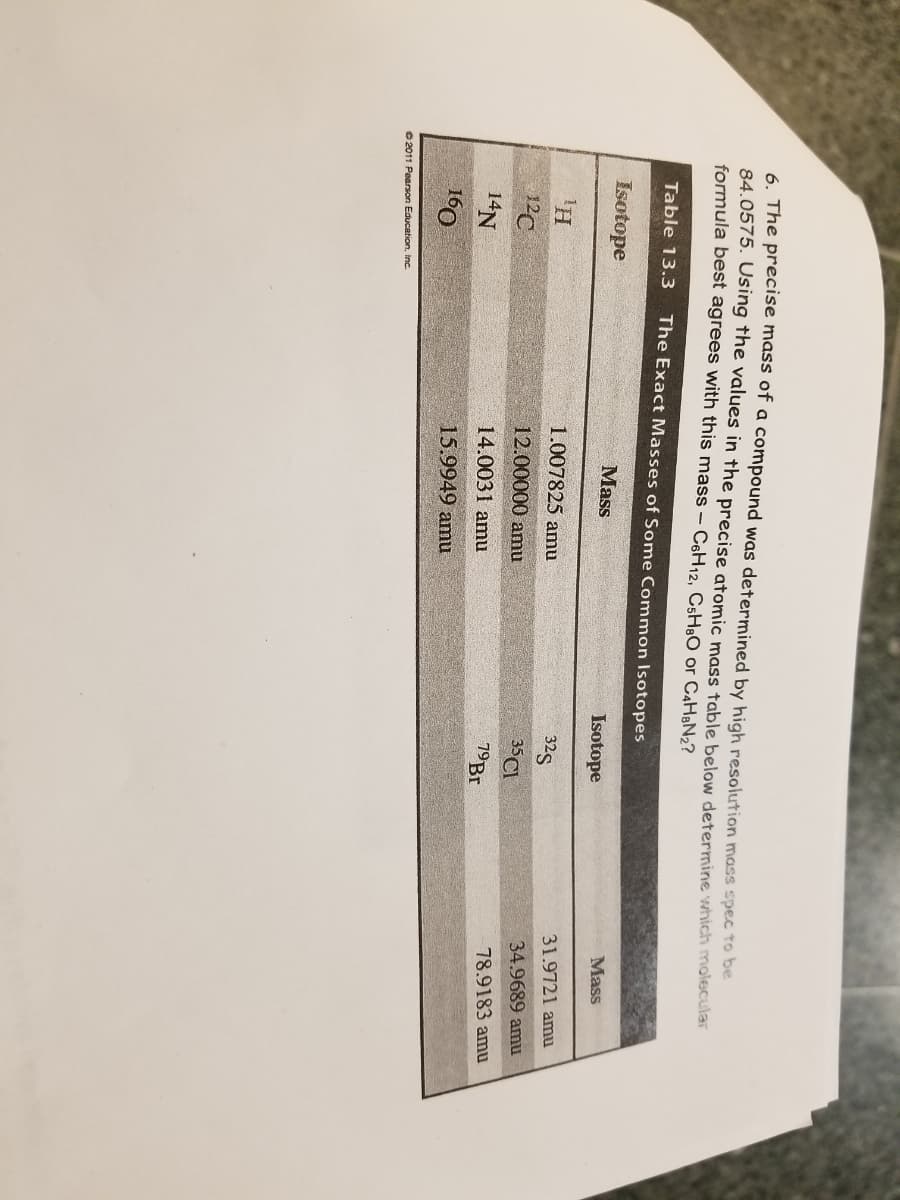
Transcribed Image Text:6. The precise mass of a compound was determined by high resolution mass spec to be
84.0575. Using the values in the precise atomic mass table below determine which molecular
formula best agrees with this mass – C6H12, CsH:O or C4H&N2?
Table 13.3
The Exact Masses of Some Common Isotopes
Mass
Isotope
Mass
Isotope
31.9721 amu
1.007825 amu
325
34.9689 amu
35CI
12C
12.00000 amu
78.9183 amu
19B.
14N
14.0031 amu
15.9949 amu
160
O 2011 Pearson Education, inc.
Expert Solution
Step 1
Given the precise mass of the compound = 84.0575 amu
We have to choose the most appropriate formula among C6H12, C5H8O, and C4H8N2.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
