6. Use Boltzmann distribution to solve this problem. A system consists of 3,000 particles that can only occupy two energy levels: a nondegen- erate ground state of 0.052 eV and a threefold degenerate excited state at 0.156 eV. If T = 900 K, (а) find the number of particles at each energy level. (b) what is the total energy of the system? ev 0,052 ev
6. Use Boltzmann distribution to solve this problem. A system consists of 3,000 particles that can only occupy two energy levels: a nondegen- erate ground state of 0.052 eV and a threefold degenerate excited state at 0.156 eV. If T = 900 K, (а) find the number of particles at each energy level. (b) what is the total energy of the system? ev 0,052 ev
Related questions
Question
100%
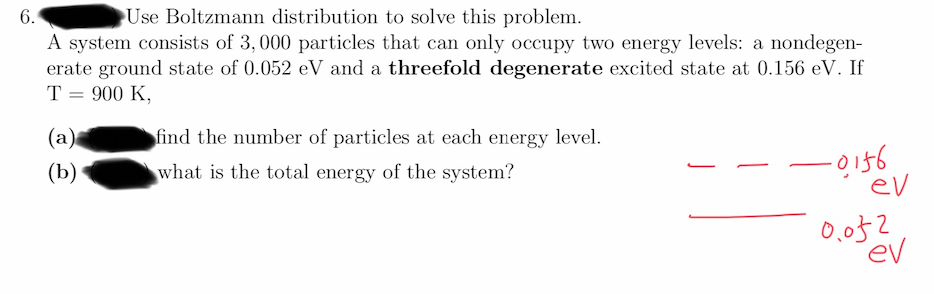
Transcribed Image Text:6.
Use Boltzmann distribution to solve this problem.
A system consists of 3, 000 particles that can only occupy two energy levels: a nondegen-
erate ground state of 0.052 eV and a threefold degenerate excited state at 0.156 eV. If
T = 900 K,
(a)
find the number of particles at each energy level.
-0156
ev
(b)
what is the total energy of the system?
0,052
ev
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps
