62. Fructose is a very sweet natural sugar that is present in honey, fruits. and fruit juices. It has a molar mass of 180.1 g/mol and a composition of 40% C. 6.7% H. and 53.3% O. Calculate the empirical formula of fructose. (Given: C 12.00 glmol; H = 1.01 g/mol: 03D 16.00 g/mol)* OA C4H403 OB C3H503 Oc.C2H20 D CH20
62. Fructose is a very sweet natural sugar that is present in honey, fruits. and fruit juices. It has a molar mass of 180.1 g/mol and a composition of 40% C. 6.7% H. and 53.3% O. Calculate the empirical formula of fructose. (Given: C 12.00 glmol; H = 1.01 g/mol: 03D 16.00 g/mol)* OA C4H403 OB C3H503 Oc.C2H20 D CH20
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter3: Calculations With Chemical Formulas And Equaitons
Section: Chapter Questions
Problem 3.18QP: Moles within Moles and Molar Mass Part 1: a How many hydrogen and oxygen atoms are present in 1...
Related questions
Question
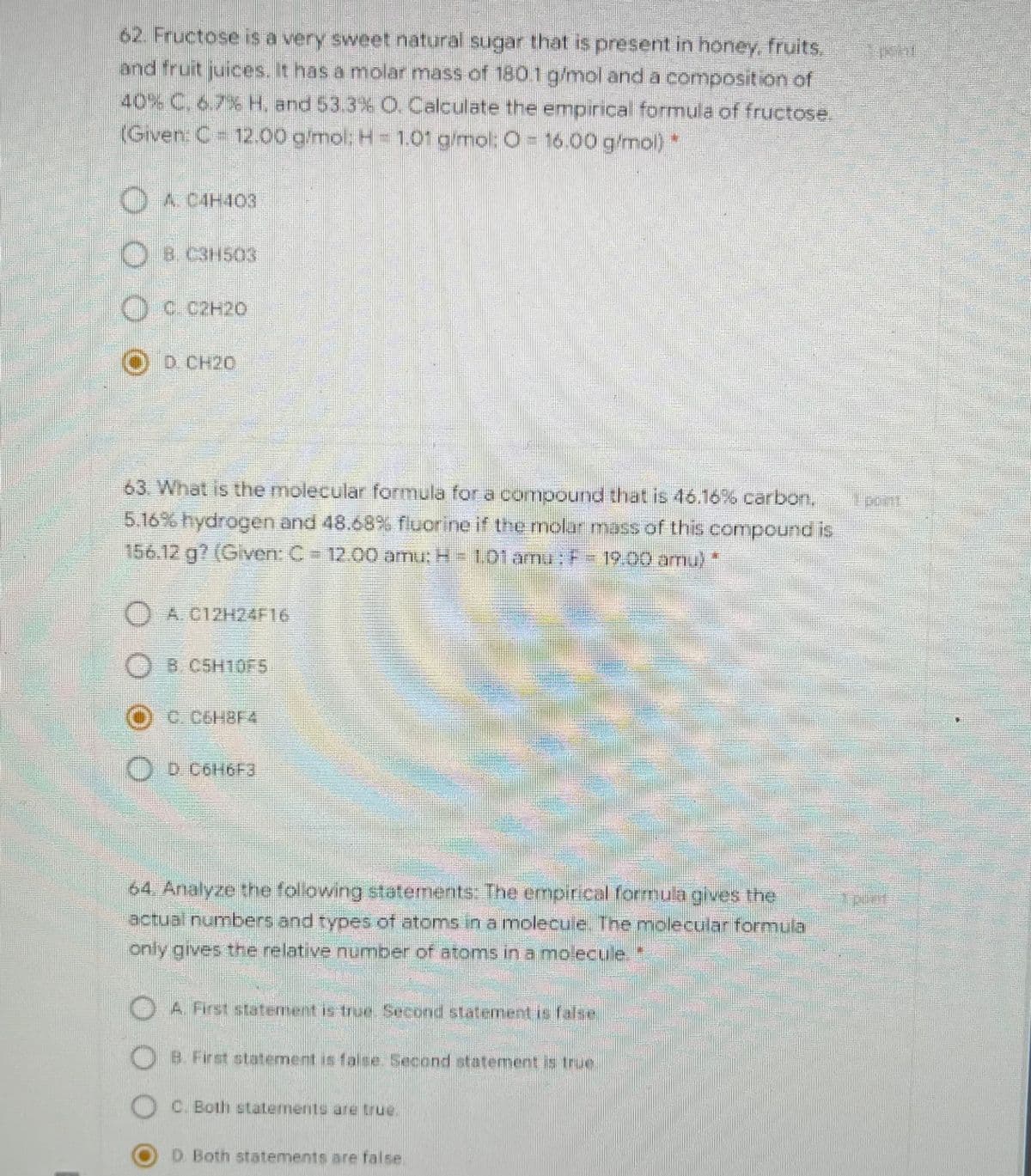
Transcribed Image Text:62. Fructose is a very sweet natural sugar that is present in honey, fruits.
and fruit juices. It has a molar mass of 1801g/mol and a composition of
40% C, 6,7% H. and 53.3% O. Calculate the empirical formula of fructose.
(Given: C 12.00 gmol: H= 101 g/mol: O= 16.00 g/mol)
O
A CAH403
OD CH20
63 What is the molecular formula for a compound that is 46.16%6 carbon, nt
5.16%hydrogen and 48.68 s fluorine if the molar mass of this compound is
156.12.g? (Given: C12.00 amu: H-totamr: 19:00 amu)
) A C12H24F16
O
B.CSH10F5
O D COH6F3
64, Analyze the folowing statements: The empirical formula gives the
actual numbers and types of atoms in a molecule.The molecular formula
only gives the relative number of atoms in a molecule*
A. First statement is true.Second statement is false
B. First statement is false Second statement is true
C. Both statLements are true.
D Both statements are false
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning