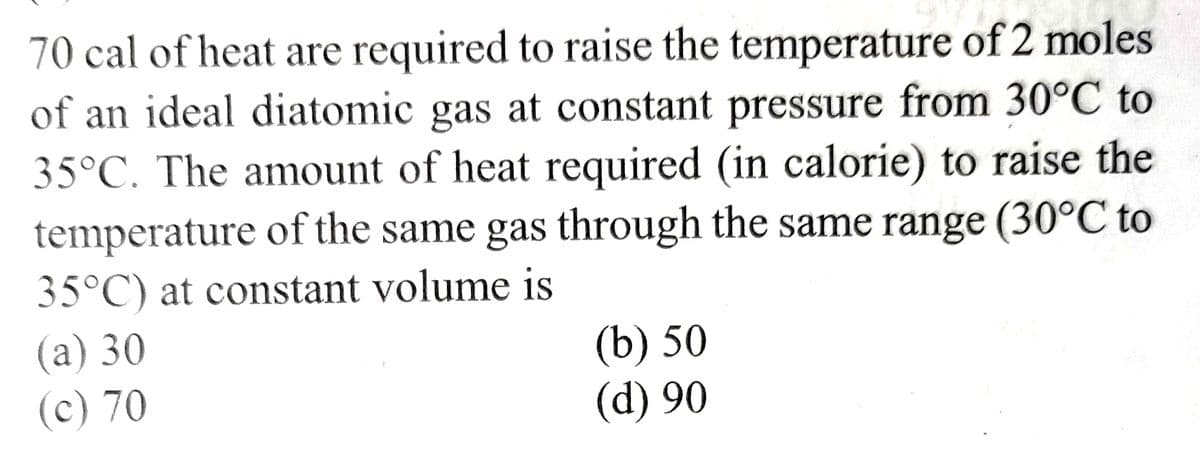
70 cal of heat are required to raise the temperature of 2 moles of an ideal diatomic gas at constant pressure from 30°C to 35°C. The amount of heat required (in calorie) to raise the temperature of the same gas through the same range (30°C to 35°C) at constant volume is (a) 30 (c) 70 (b) 50 (d) 90
Q: Figure shows an irregular block of material of refractive index √√2. A ray of light strikes the face…
A: We are aware that the illustration depicts an asymmetrical block of material with a refractive…
Q: ork concentration profile c(r) of molecules in a centrifuge, which is Nelson's Eq. A sample is…
A: In a rotating frame, there exist centrifugal force and centrifugal potential energy. The molecules…
Q: Aluminium is trivalent with atomic weight 27 g/mole and density 2.7×10³ kg/m³, whilst the mean free…
A: Given that: Atomic weight, M=27 g/moleDenisty, ρ=2.7×103 kg/m3Mean time, T=4×10-14 sLength of the…
Q: Show that the divergence of a curl is always zero and verify your answer using the example = 3y³z²î…
A:
Q: 1. A particle at rest decays into two corfu particles, each with a mass of 200.0 MeV/c². One of the…
A: One particle is decayed and it forms two Corfu particle so we will calculate the mass of the…
Q: J04
A: Given that-Wavelength 'λ'= 40.0 mSpeed of propagation, v= 5.0 m/sTime 't'= 1 minutet= 60 secWe know…
Q: A satellite is in a circular orbit at an altitude of 250 km above the earth's surface. If an onboard…
A: Concept: To determine the Altitude of the satellite in the new orbit apogee we will have to…
Q: I= Problem 2: A 5.0-mm-diameter proton beam carries a total current of = 1.5 mA. The current density…
A: Solution: The diameter of the proton beam is, d=5 mm =5×10-3 mm The total current of the proton…
Q: radialed in one Calculate energy by a black body surface of area 100 cm ² when it is maintained at…
A: We need to compute-Energy radiated (Q)=?The data given as-Surface area (A)=100 cm2A=10-2…
Q: A person brings a bathroom scale onto an elevator. For each of the following situations, eval- uate…
A:
Q: What frequency of sound traveling in air at 40 °C has a wavelength of 0.50 m?
A: We have-Wavelength,λ= 0.50 mTemperature, T= 40°CWe know that-vsound in air= 331.4 + 0.6 Tcsubstitute…
Q: A rectangular coil with a length of 8.0 cm and a width of 4.0 cm and 250 turns is placed in a…
A: We have following values-Length, l= 8.0 cm=8×10-2 mWidth, b=4.0 cm=4×10-2 mNumber of turns, N= 250…
Q: 19 Exg Find the F.T. of the rectangular pulse (gate function) EX f(t) = A rect ( =) = ATT (/), I is…
A:
Q: A slit of width d is placed in front of a lens of focal length 0.5 m and is illuminated normally…
A: We are aware that a slit of width d is positioned in front of a lens with a focal length of 0.5 m…
Q: 4. What is the percentage strength (v/v) if 225 g of a liquid having a specific gravity of 0.8 is…
A: We know that specific gravity is the density of liquid divided by density of water at 4 degree. We…
Q: = Let y₁(x, t) = A₁ cos(k₁x - w₁t) and y₂(x, t) A2 cos(k₂x wat) be two solutions to the wave…
A:
Q: Satellite A 120° Satellite B Problem 2 The following figure shows two satellites in Earth orbits:…
A: Given Data: To Determine the perigee altitude of the elliptical orbit so that Satellites A and B…
Q: 7. Consider a thin positive lens L₁ with a focal length of fi. If a second lens L2 with a focal…
A: Determine, The magnification of the final image does not change with respect to position of the…
Q: A 60 watt electric bulb loses its energy entirely by radiation from the surface of filament. If the…
A: We need to compute-Temperature of filament (T)=?The data given as-Power dQdt=60 wattArea of filament…
Q: Based on what you know about Kirchhoff’s laws of spectroscopy, explain how the Sun’s light can be…
A: Kirchhoff gave 3 laws of spectroscopy. The first law states a continuous spectrum is produced by a…
Q: A 12 pF capacitor is connected to a 50 V battery. How much electrostatic energy is stored in the…
A: We need to compute-(i) Electrostatic energy in 12pF capacitor (U)=?(ii) Total charge stored in…
Q: Find RMS velocity of three molecule having velocities 10 km/s, 20 km / s, 30 km/s.
A: We need to compute-RMS velocity (C2)=?The data given as-C1=10 km/sC2=20 km/sC3=30 km/s
Q: A. State Frank-Condon principal B. State Born- Oppenheimer approximation
A: Franck Cordon principle: The F-C principle states that the electron's transition occur so rapid that…
Q: 6. Solve the following differential equations: 38 38 dy dx (a) + dy (b) du dx xy 4+x² 2x 4y² – 2y²…
A:
Q: 0.003 0.005 Solve for: arithmetic 0.30 mean diameter.
A: Solution: The arithmetic mean of n numbers is given by the following relation: m=∑xinHere, ∑xi-sum…
Q: An artificial satellite is moving in a circular orbit around the Earth with a speed equal to half…
A: Considering that an artificial satellite is orbiting the Earth in a circular fashion at a speed that…
Q: A metal strip 7.00 cm long, 0.82 cm wide, and 0.71 mm thick moves with constant velocity (v) through…
A:
Q: Exs Find the F.T of the signal shown below ir cos 0) f(t) Amin (a) Na DOVE WELK
A:
Q: A 3.00 mF capacitor is connected to a standard electrical outlet (AVrms = 120 V; f = 60.0 Hz).…
A: Given: In this question, the given details are, Here, a 3.00 mF capacitor is connected to a standard…
Q: Figure shows an irregular block of material of refractive index √2. A ray of light strikes the face…
A: We are aware that the illustration depicts an asymmetrical block of material with a refractive…
Q: After losing a game of paper-rock-scissors to Philopater, Michelle takes a smaller craft down to…
A: Disclaimer: Since you have asked multiple question, we will solve the first question for you. If you…
Q: The ammeter shown in the figure below reads 2.41 A. Find 1₁, 12, and E. (Assume R = 7.35 2.) 4₁ = A…
A: For calculation of I1 and I2 we need to apply kirchoff's voltage loop law in loop…
Q: . If collision between the block and the incline is completely elastic, then the vertical (upward)…
A: Here, we know that if the collision between the block and the incline is entirely elastic, then we…
Q: Consider a 100-cm-long string fixed at both ends. The string can vibrate in standing wave pattern.…
A: We are given a string which is being fixed at both ends. Hence there is nodes at both ends. The…
Q: v m/s A ball of mass 0.2 kg rests on a vertical post of height 5 m. A bullet of mass 0.01 kg,…
A: We are aware that a 0.2 kilogramme ball rests on a 5 m tall vertical pole. a bullet with a…
Q: The ratio A. B. C. D. thermal capacity of a sample of copper specific heat capacity of copper does…
A:
Q: A 60 watt electric bulb loses its energy entirely by radiation from the surface of filament. If the…
A:
Q: acceleration force energy power SI Units m [22] [N] [J] [W] Natural Units 1.2×10-¹7 [1] m 15 [kg] m…
A:
Q: ANSWER COMPLETELY FOR UPVOTE a. When do we say that the body is accelerating? b. What's the…
A: (a). When the velocity of the body change with time then we say that body is accelerating. Example :…
Q: 1. A boat can be rowed at Think & Prepare 1. In all the questions below, think about motion from the…
A: Disclaimer: “Since you have asked posted a question with multiple sub-parts, we will solve the first…
Q: A 6.1 kg solid metal sphere has a density of 2600 kg/m^3. It is hung from a cord and completely…
A: Given: In this question, the given details are, A 6.1 kg solid metal sphere has a density of 2600…
Q: Saturn has an angular size of 16”, and an observed Synodic Period of 1.035yrs. Saturn’s moon, Titan…
A: Given , The Synodic Period of Saturn is 1.035 years. Using Sidereal Period of Saturn, we have to…
Q: d. Determine the velocity of the person relative to the water (with direction) e. Determine the…
A: Dear student as per the guidelines I will be answering only parts d and e. Given, Penny drops leaf…
Q: A 6400-kg elevator has a maximum acceleration of 2.5 m/s². Calculate the minimum diameter steel…
A: Given that-Mass of elevator, m= 6400 kgMagnitude of acceleration, a= 2.5 m/sec2As we know that-Net…
Q: Joe is 1.79 m high. What is the minimal size of a plane mirror where he can see a full view of…
A: Solution:-Given thatHeight of Joe (h)=1.79 m
Q: Two satellites A and B have masses mand 2m respectively. A is in a circular orbit of radius R and B…
A: Two satellites, A and B, are known to have masses of m and 2 m, respectively. Around the earth, A is…
Q: 2. An iceberg (y=9 kN/m3) floats in ocean water (y = 10 kN/m3) with 700 m³ of the iceberg protruding…
A:
Q: . Find RMS velocity of three molecule having velocities 10 km / s, 20 km / s, 30 km/s.
A: We are given the velocities of 3 molecules. The rms velocity of n number of molecules is given as…
Q: Consider the circuit in figure 4. a) Isolate R₁, and draw the Thevenin Equivalent circuit at…
A:
Q: An archer shoots a 0.024-kg arrow at a target with a speed of 54 m/s. When it hits the target, it…
A: We are given the initial velocity. We are also given the stopping distance. We first find the…
Step by step
Solved in 3 steps
