70 Redox Reactions Part A: Other Redox Reactions in Acidic and Basic Solution- Experiment Oxidizing Reducing Agent Observations Kmn O t Hz SOy-po color change (Dark Purple) Agent Number 2t + Fesou clear Fe Mnon 34 24 Balanced Net +7 lediMnon Ionic Equation K: Fe Fe? Y Kmnoy +NAOH nO Chemge (Derk purphe) 2 M.OM +Fesoy Broren Fe 43 +Mnbe BasO MhOn 7 2401MM+3 F2MnAt3 Fet +40H Balanced Net Ionic Equation K. m nowt H2 SOu ho Change CDerk Porple 3 2 Mnou2 Ka Ou 7 chear +7 +3 2. 24 -Coz lo C07 2 MAOy +52042M Balanced 16 H Net Ionic Equation 71 Chemistry I Laboratory Manual, 2017 Revision Experiment Oxidizing Reducing Agent Tamber K2 Cra Op t Ha2So4he charye Observations Number Agent NaNo, Cs) gt blne N Balanced 3NA 3NOt 26 43o H Net Ionic Equation S 9 Fe Sou -> ht Balanced Net Ionic Equation KI H NOs Clear Hot ater ligkyelarw Balanced Net lonic Equation
70 Redox Reactions Part A: Other Redox Reactions in Acidic and Basic Solution- Experiment Oxidizing Reducing Agent Observations Kmn O t Hz SOy-po color change (Dark Purple) Agent Number 2t + Fesou clear Fe Mnon 34 24 Balanced Net +7 lediMnon Ionic Equation K: Fe Fe? Y Kmnoy +NAOH nO Chemge (Derk purphe) 2 M.OM +Fesoy Broren Fe 43 +Mnbe BasO MhOn 7 2401MM+3 F2MnAt3 Fet +40H Balanced Net Ionic Equation K. m nowt H2 SOu ho Change CDerk Porple 3 2 Mnou2 Ka Ou 7 chear +7 +3 2. 24 -Coz lo C07 2 MAOy +52042M Balanced 16 H Net Ionic Equation 71 Chemistry I Laboratory Manual, 2017 Revision Experiment Oxidizing Reducing Agent Tamber K2 Cra Op t Ha2So4he charye Observations Number Agent NaNo, Cs) gt blne N Balanced 3NA 3NOt 26 43o H Net Ionic Equation S 9 Fe Sou -> ht Balanced Net Ionic Equation KI H NOs Clear Hot ater ligkyelarw Balanced Net lonic Equation
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter24: Coulometry
Section: Chapter Questions
Problem 24.9QAP
Related questions
Question
Part A: Other
Give a balanced net ionic equation for all six reactions (if possible). please show work.
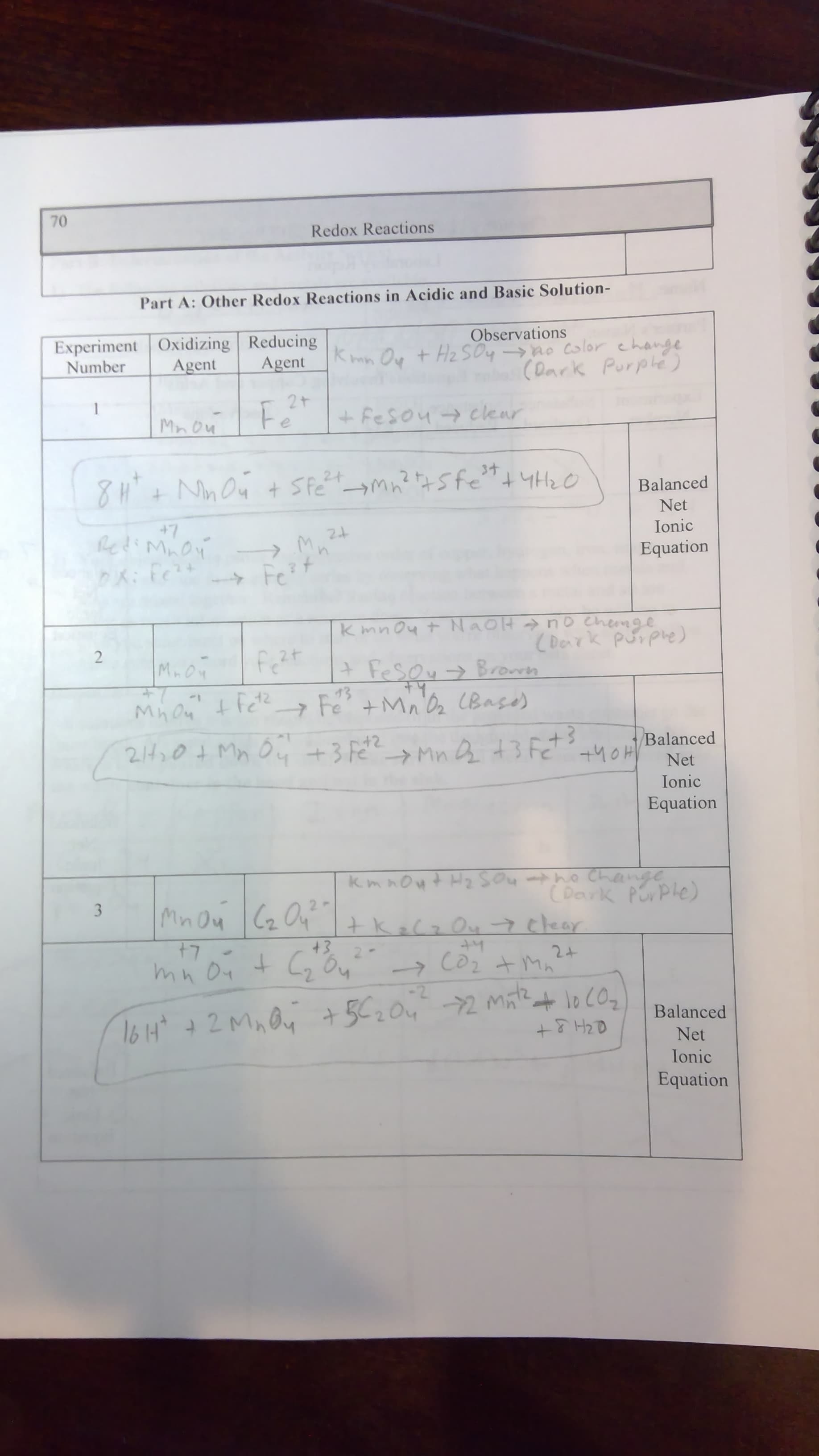
Transcribed Image Text:70
Redox Reactions
Part A: Other Redox Reactions in Acidic and Basic Solution-
Experiment Oxidizing Reducing
Agent
Observations
Kmn O t Hz SOy-po color change
(Dark Purple)
Agent
Number
2t
+ Fesou clear
Fe
Mnon
34
24
Balanced
Net
+7
lediMnon
Ionic
Equation
K: Fe
Fe?
Y
Kmnoy +NAOH nO Chemge
(Derk purphe)
2
M.OM
+Fesoy Broren
Fe
43
+Mnbe BasO
MhOn
7
2401MM+3 F2MnAt3 Fet
+40H
Balanced
Net
Ionic
Equation
K. m nowt H2 SOu
ho Change
CDerk Porple
3
2
Mnou2 Ka Ou 7 chear
+7
+3
2.
24
-Coz
lo C07
2 MAOy +52042M
Balanced
16 H
Net
Ionic
Equation
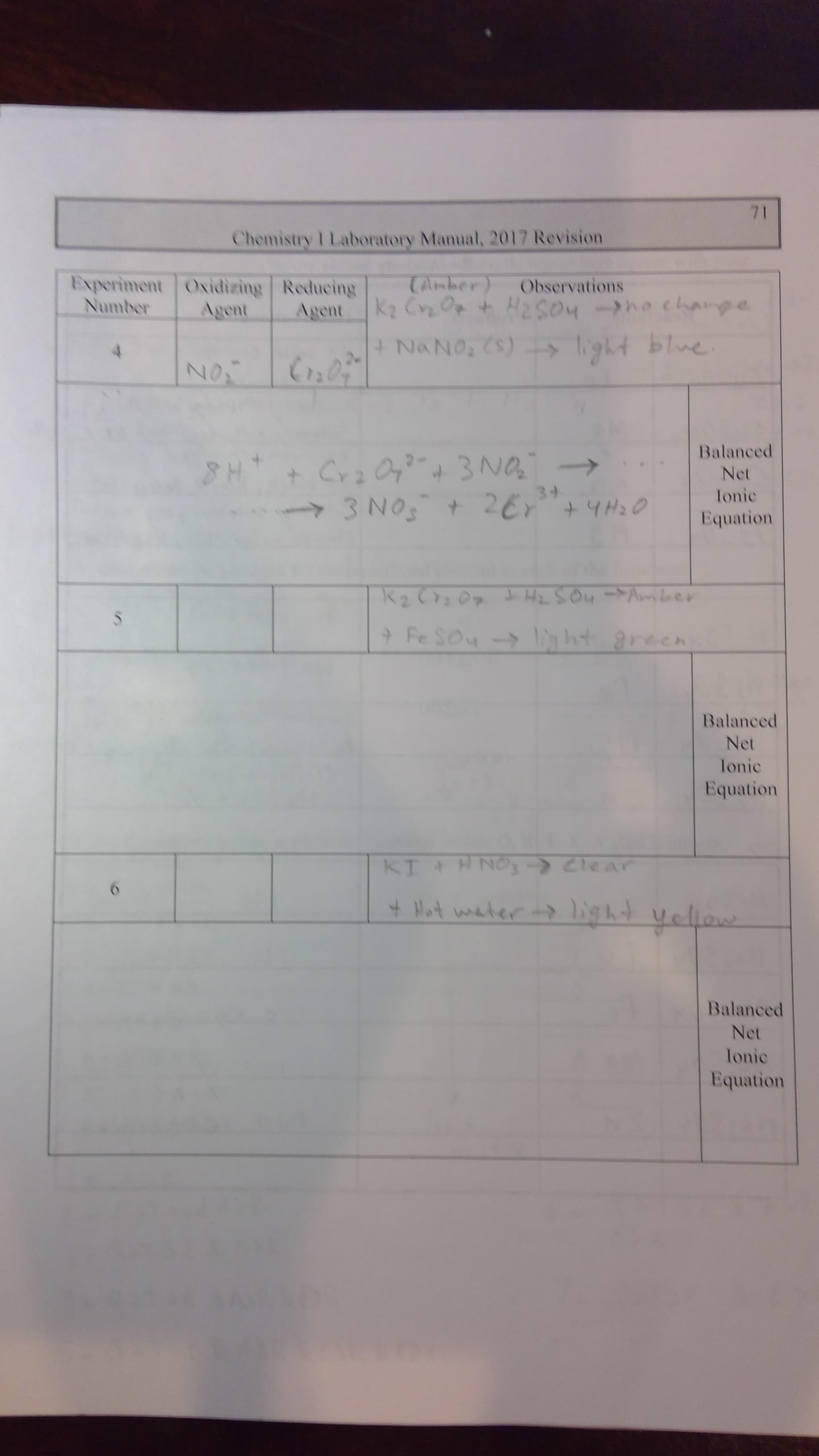
Transcribed Image Text:71
Chemistry I Laboratory Manual, 2017 Revision
Experiment Oxidizing Reducing
Agent
Tamber
K2 Cra Op t Ha2So4he charye
Observations
Number
Agent
NaNo, Cs) gt blne
N
Balanced
3NA
3NOt 26 43o
H
Net
Ionic
Equation
S
9 Fe Sou -> ht
Balanced
Net
Ionic
Equation
KI H NOs Clear
Hot ater ligkyelarw
Balanced
Net
lonic
Equation
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
