73. CH3OH can be synthesized by the reaction: CH3OH(g) CO(g) + 2 H2(g) What volume of H2 gas (in L), at 748 mmHg and 86 °C, is re- quired to synthesize 25.8 g CH3OH? How many liters of CO gas, measured under the same conditions, are required?
73. CH3OH can be synthesized by the reaction: CH3OH(g) CO(g) + 2 H2(g) What volume of H2 gas (in L), at 748 mmHg and 86 °C, is re- quired to synthesize 25.8 g CH3OH? How many liters of CO gas, measured under the same conditions, are required?
Introductory Chemistry: A Foundation
8th Edition
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter13: Gases
Section: Chapter Questions
Problem 142AP: Consider the following unbalanced chemical equation: ^Sfr) + O2(g) —? Cu2O(s) + SO2(g) What volume...
Related questions
Question
#73
Please solve on a sheet of paper.
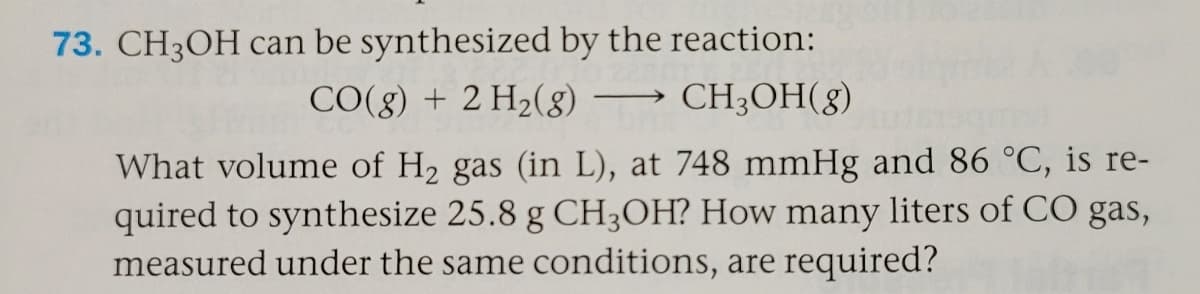
Transcribed Image Text:73. CH3OH can be synthesized by the reaction:
CO(g) + 2 H2(g) –→ CH3OH(g)
What volume of H2 gas (in L), at 748 mmHg and 86 °C, is re-
quired to synthesize 25.8 g CH3OH? How many liters of CO gas,
measured under the same conditions, are required?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
