7E R-134a vapor enters into a turbine at 250 psia and PF. The temperature of R-134a is reduced to 20°F in this ine while its specific entropy remains constant. Determine change in the enthalpy of R-134a as it passes through the ine.
7E R-134a vapor enters into a turbine at 250 psia and PF. The temperature of R-134a is reduced to 20°F in this ine while its specific entropy remains constant. Determine change in the enthalpy of R-134a as it passes through the ine.
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Question
Show all work. Please show the interpolation method.
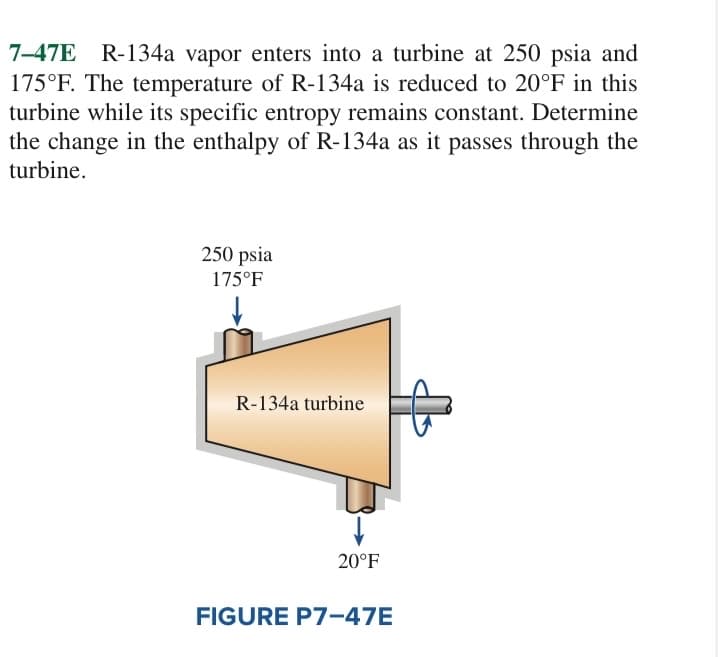
Transcribed Image Text:7-47E
R-134a vapor enters into a turbine at 250 psia and
175°F. The temperature of R-134a is reduced to 20°F in this
turbine while its specific entropy remains constant. Determine
the change in the enthalpy of R-134a as it passes through the
turbine.
250 psia
175°F
R-134a turbine
20°F
FIGURE P7-47E
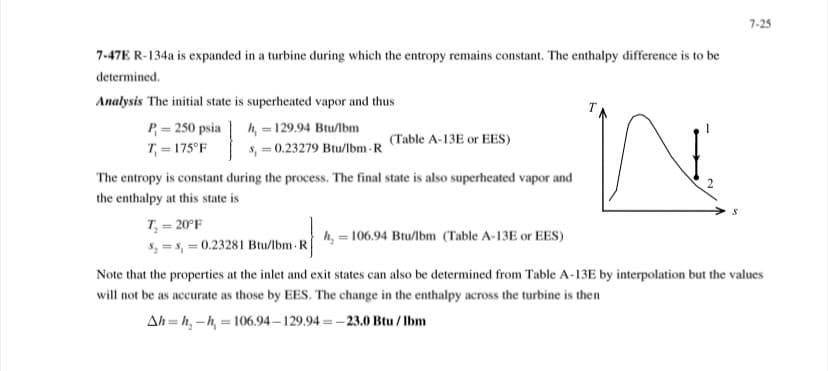
Transcribed Image Text:7-25
7-47E R-134a is expanded in a turbine during which the entropy remains constant. The enthalpy difference is to be
determined.
Analysis The initial state is superheated vapor and thus
h, = 129.94 Btu/lbm
s, = 0.23279 Btu/lbm - R
P = 250 psia
(Table A-13E or EES)
T = 175°F
The entropy is constant during the process. The final state is also superheated vapor and
the enthalpy at this state is
T = 20°F
h, = 106.94 Btu/lbm (Table A-13E or EES)
5, = s, = 0.23281 Btu/lbm -R
Note that the properties at the inlet and exit states can also be determined from Table A-13E by interpolation but the values
will not be as accurate as those by EES. The change in the enthalpy across the turbine is then
Ah = h, -h, = 106.94 – 129.94 = –23.0 Btu / lbm
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY