8 Demonstrate your knowledge of Carbon and Hydrogen types on the structure of that same bloody molecule listed below. Indicate whether the "C types are (1°, 2°, 3°, 4° or N/A). 99 and H CH2 H2N-CH2 15 14 1 НАС —0 4 3 5 10 CH2-C 2 12 11 6 7 CH3 13 3 H 1. с _ 3. C H N/A 2. C H 2 H_N/A 4. С С 4 5. С H NIA H 6. C 3 2 7. с 2 H 8. C 9. С 2 H H 2 10. C H MA 11. С H H N/A 12. C 13. С I H 3 14. С 3 н_ 15. C H
8 Demonstrate your knowledge of Carbon and Hydrogen types on the structure of that same bloody molecule listed below. Indicate whether the "C types are (1°, 2°, 3°, 4° or N/A). 99 and H CH2 H2N-CH2 15 14 1 НАС —0 4 3 5 10 CH2-C 2 12 11 6 7 CH3 13 3 H 1. с _ 3. C H N/A 2. C H 2 H_N/A 4. С С 4 5. С H NIA H 6. C 3 2 7. с 2 H 8. C 9. С 2 H H 2 10. C H MA 11. С H H N/A 12. C 13. С I H 3 14. С 3 н_ 15. C H
Chapter3: Organic Compounds: Alkanes And Their Stereochemistry
Section3.SE: Something Extra
Problem 50AP: Formaldehyde, H2C=O, is known to all biologists because of its usefulness as a tissue preservative....
Related questions
Question
Check please! :)
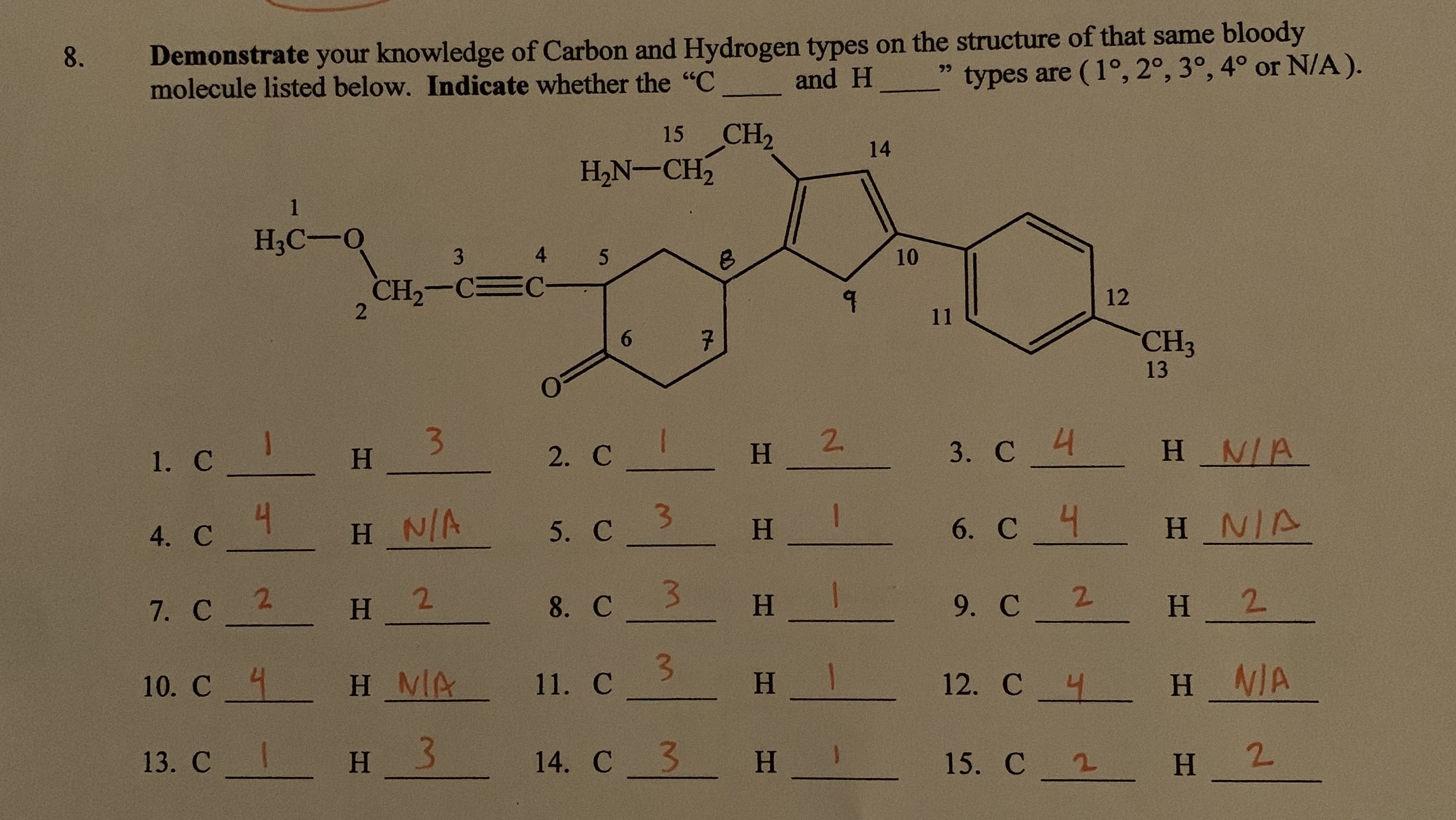
Transcribed Image Text:8
Demonstrate your knowledge of Carbon and Hydrogen types on the structure of that same bloody
molecule listed below. Indicate whether the "C
types are (1°, 2°, 3°, 4° or N/A).
99
and H
CH2
H2N-CH2
15
14
1
НАС —0
4
3
5
10
CH2-C
2
12
11
6
7
CH3
13
3
H
1. с _
3. C H N/A
2. C
H 2
H_N/A
4. С
С 4
5. С
H NIA
H
6. C
3
2
7. с 2
H
8. C
9. С 2
H
H 2
10. C H MA
11. С
H
H N/A
12. C
13. С I
H 3
14. С 3
н_
15. C
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
