8. A solution containing 1.00 g of an unknown nonelectrolyte liquid and 9.00 g water has a freezing point of -3.33 °C. The Kr= 1.86 °C/m for water. Calculate the molar mass of the unknown liquid in g/mol.
8. A solution containing 1.00 g of an unknown nonelectrolyte liquid and 9.00 g water has a freezing point of -3.33 °C. The Kr= 1.86 °C/m for water. Calculate the molar mass of the unknown liquid in g/mol.
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter13: Solutions And Their Behavior
Section: Chapter Questions
Problem 71GQ: An aqueous solution containing 10.0 g of starch per liter has an osmotic pressure of 3.8 mm Hg at 25...
Related questions
Question
Please explain 8. How can I find molar mass?
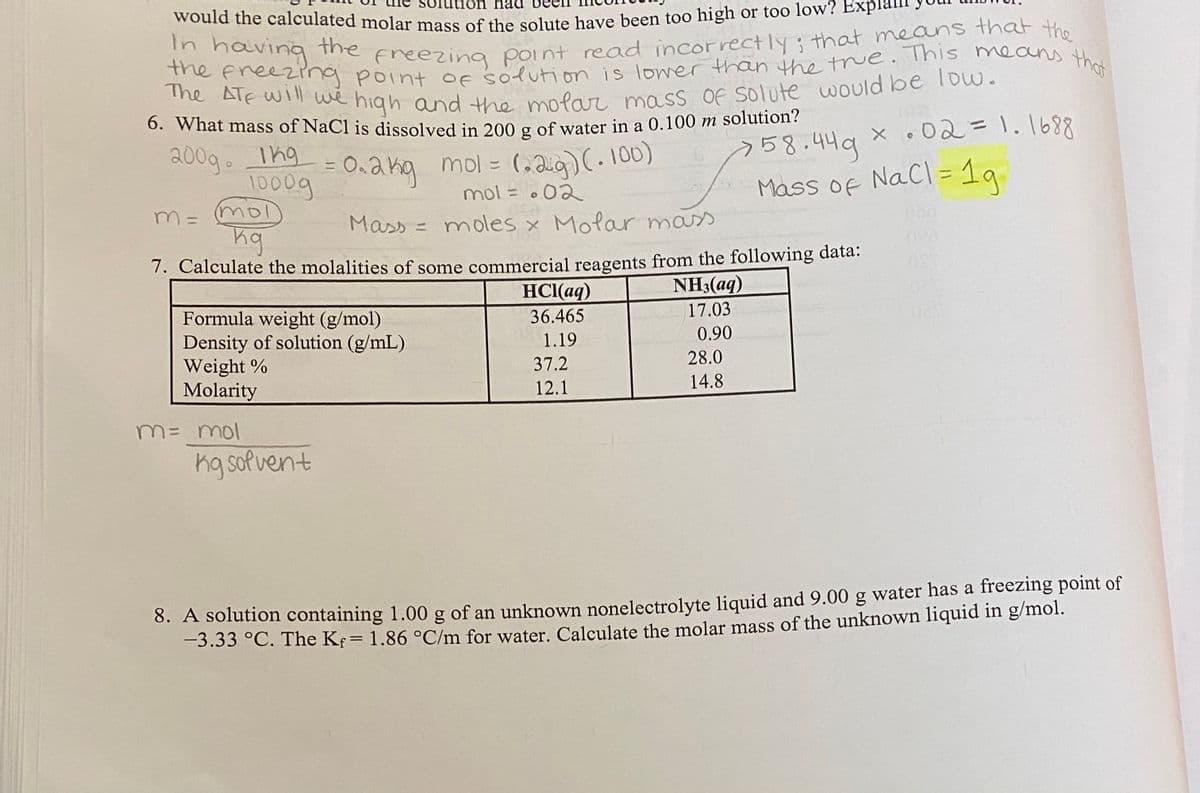
Transcribed Image Text:6. What mass of NaCl is dissolved in 200 g of water in a 0.100 m solution?
the free2ing point of solution is lower than the true. This means that
The ATF will we high and the molar mass Of solute would be loww.
would the calculated molar mass of the solute have been too high or too low? Bxplam
In having
the
T reezrng point of solution is lowerr than the tue. This means tha
he Ale wl we high and the mofan ma ss of solute would be Tow.
Freezing Point read incorrectly; that means that the
6. What mass of NaCl is dissolved in 200 g of water in a 0.100 m solution?
%3D
258.449 x .02=1.1698
う58.44g
200g. Thg = 0.akg
0.akg mol = (aa)(.100)
1000g
mol
Mass of Nacl= 19
mol = o02
%3D
Mass = moles × Molar mass
%3D
1. Calculate the molalities of some commercial reagents from the following data:
HCl(aq)
36.465
NH3(aq)
17.03
Formula weight (g/mol)
Density of solution (g/mL)
Weight %
Molarity
1.19
0.90
37.2
28.0
12.1
14.8
M= mol
Kg sofvent
8. A solution containing 1.00 g of an unknown nonelectrolyte liquid and 9.00 g water has a freezing point of
-3.33 °C. The Kf= 1.86 °C/m for water. Calculate the molar mass of the unknown liquid in g/mol.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning