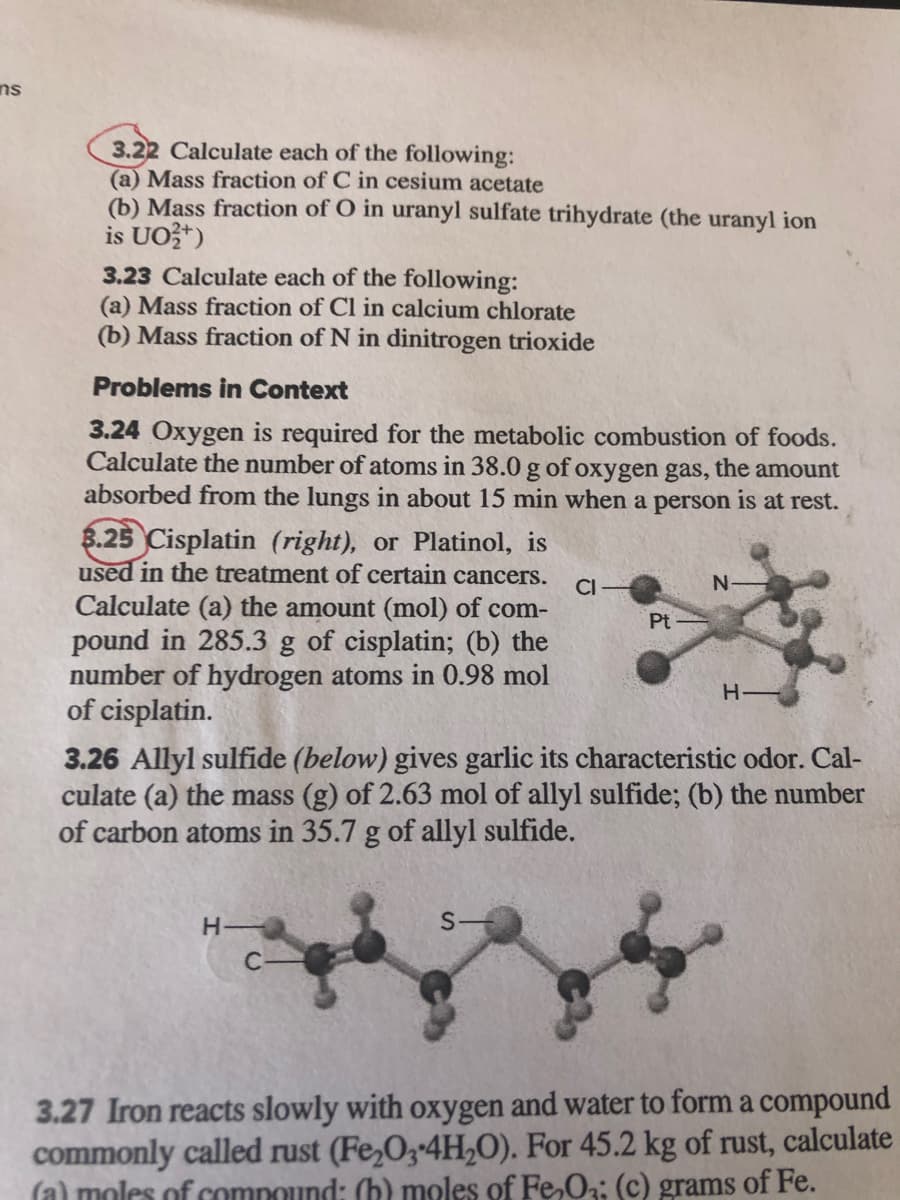
8.25 Cisplatin (right), or Platinol, is used in the treatment of certain cancers. CI- N- Calculate (a) the amount (mol) of com- pound in 285.3 g of cisplatin; (b) the number of hydrogen atoms in 0.98 mol of cisplatin. Pt
8.25 Cisplatin (right), or Platinol, is used in the treatment of certain cancers. CI- N- Calculate (a) the amount (mol) of com- pound in 285.3 g of cisplatin; (b) the number of hydrogen atoms in 0.98 mol of cisplatin. Pt
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.15QAP
Related questions
Question
question 25 please
Transcribed Image Text:ns
3.22 Calculate each of the following:
(a) Mass fraction of C in cesium acetate
(b) Mass fraction of O in uranyl sulfate trihydrate (the uranyl ion
is UO?*)
3.23 Calculate each of the following:
(a) Mass fraction of Cl in calcium chlorate
(b) Mass fraction of N in dinitrogen trioxide
Problems in Context
3.24 Oxygen is required for the metabolic combustion of foods.
Calculate the number of atoms in 38.0 g of oxygen gas, the amount
absorbed from the lungs in about 15 min when a person is at rest.
8.25 Cisplatin (right), or Platinol, is
used in the treatment of certain cancers.
Calculate (a) the amount (mol) of com-
pound in 285.3 g of cisplatin; (b) the
number of hydrogen atoms in 0.98 mol
of cisplatin.
CI
Pt
H-
3.26 Allyl sulfide (below) gives garlic its characteristic odor. Cal-
culate (a) the mass (g) of 2.63 mol of allyl sulfide; (b) the number
of carbon atoms in 35.7 g of allyl sulfide.
H
S-
3.27 Iron reacts slowly with oxygen and water to form a compound
commonly called rust (Fe,03-4H,0). For 45.2 kg of rust, calculate
(a) moles of compound: (b) moles of Fe,O: (c) grams of Fe.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

