86 In an industrial process the volume of 25.0 mol of a monatomic ideal gas is reduced at a uniform rate from 0.616 m to 0.308 m³ in 2.00 h while its temperature is increased at a uniform rate from 27.0°C to 450°C. Throughout the process, the gas passes through thermodynamic equilibrium states. What are (a) the cumulative work done on the gas, (b) the cumulative energy absorbed by the gas as heat, and (c) the molar specific heat for the process? (Hint: To evaluate the integral for the work, you might use ав - bА a + bx dx A + Bx bx B B? - In(A + Bx), an indefinite integral.) Suppose the process is replaced with a two- step process that reaches the same final state. In step 1, the gas volume is reduced at constant temperature, and in step 2 the tempera- ture is increased at constant volume. For this process, what are (d) the cumulative work done on the gas, (e) the cumulative energy absorbed by the gas as heat, and (f) the molar specific heat for the process?
86 In an industrial process the volume of 25.0 mol of a monatomic ideal gas is reduced at a uniform rate from 0.616 m to 0.308 m³ in 2.00 h while its temperature is increased at a uniform rate from 27.0°C to 450°C. Throughout the process, the gas passes through thermodynamic equilibrium states. What are (a) the cumulative work done on the gas, (b) the cumulative energy absorbed by the gas as heat, and (c) the molar specific heat for the process? (Hint: To evaluate the integral for the work, you might use ав - bА a + bx dx A + Bx bx B B? - In(A + Bx), an indefinite integral.) Suppose the process is replaced with a two- step process that reaches the same final state. In step 1, the gas volume is reduced at constant temperature, and in step 2 the tempera- ture is increased at constant volume. For this process, what are (d) the cumulative work done on the gas, (e) the cumulative energy absorbed by the gas as heat, and (f) the molar specific heat for the process?
Physics for Scientists and Engineers, Technology Update (No access codes included)
9th Edition
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter21: The Kinetic Theory Of Gases
Section: Chapter Questions
Problem 21.51AP: A certain ideal gas has a molar specific heat of Cv = 72R. A 2.00-mol sample of the gas always...
Related questions
Question
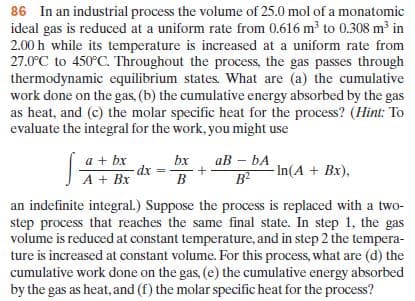
Transcribed Image Text:86 In an industrial process the volume of 25.0 mol of a monatomic
ideal gas is reduced at a uniform rate from 0.616 m to 0.308 m³ in
2.00 h while its temperature is increased at a uniform rate from
27.0°C to 450°C. Throughout the process, the gas passes through
thermodynamic equilibrium states. What are (a) the cumulative
work done on the gas, (b) the cumulative energy absorbed by the gas
as heat, and (c) the molar specific heat for the process? (Hint: To
evaluate the integral for the work, you might use
ав - bА
a + bx
dx
A + Bx
bx
B
B?
- In(A + Bx),
an indefinite integral.) Suppose the process is replaced with a two-
step process that reaches the same final state. In step 1, the gas
volume is reduced at constant temperature, and in step 2 the tempera-
ture is increased at constant volume. For this process, what are (d) the
cumulative work done on the gas, (e) the cumulative energy absorbed
by the gas as heat, and (f) the molar specific heat for the process?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 7 images

Recommended textbooks for you

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning