A 0.0200 M aqueous solution of sodium sulfate solution was prepared in a 50.00-mL volumetric flask. Then 10.00 mL of this solution was transferred to a 100.00-mL volumetric flask and distilled water was added to it until the calibration mark was reached. Then the final solution was homogenized. What was the dilution factor? O a. 2 O b. These answers are all wrong O c. one-tenth (0.1) O d. 10.00 Oe. It cannot be determined because the molarity of the final solution is unknown
A 0.0200 M aqueous solution of sodium sulfate solution was prepared in a 50.00-mL volumetric flask. Then 10.00 mL of this solution was transferred to a 100.00-mL volumetric flask and distilled water was added to it until the calibration mark was reached. Then the final solution was homogenized. What was the dilution factor? O a. 2 O b. These answers are all wrong O c. one-tenth (0.1) O d. 10.00 Oe. It cannot be determined because the molarity of the final solution is unknown
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter12: Solutions
Section: Chapter Questions
Problem 12.104QE: A 10.00-mL sample of a 24.00% solution of ammonium bromide (NH4Br) requires 23.41 mL of 1.200 molar...
Related questions
Question
Transcribed Image Text:stion
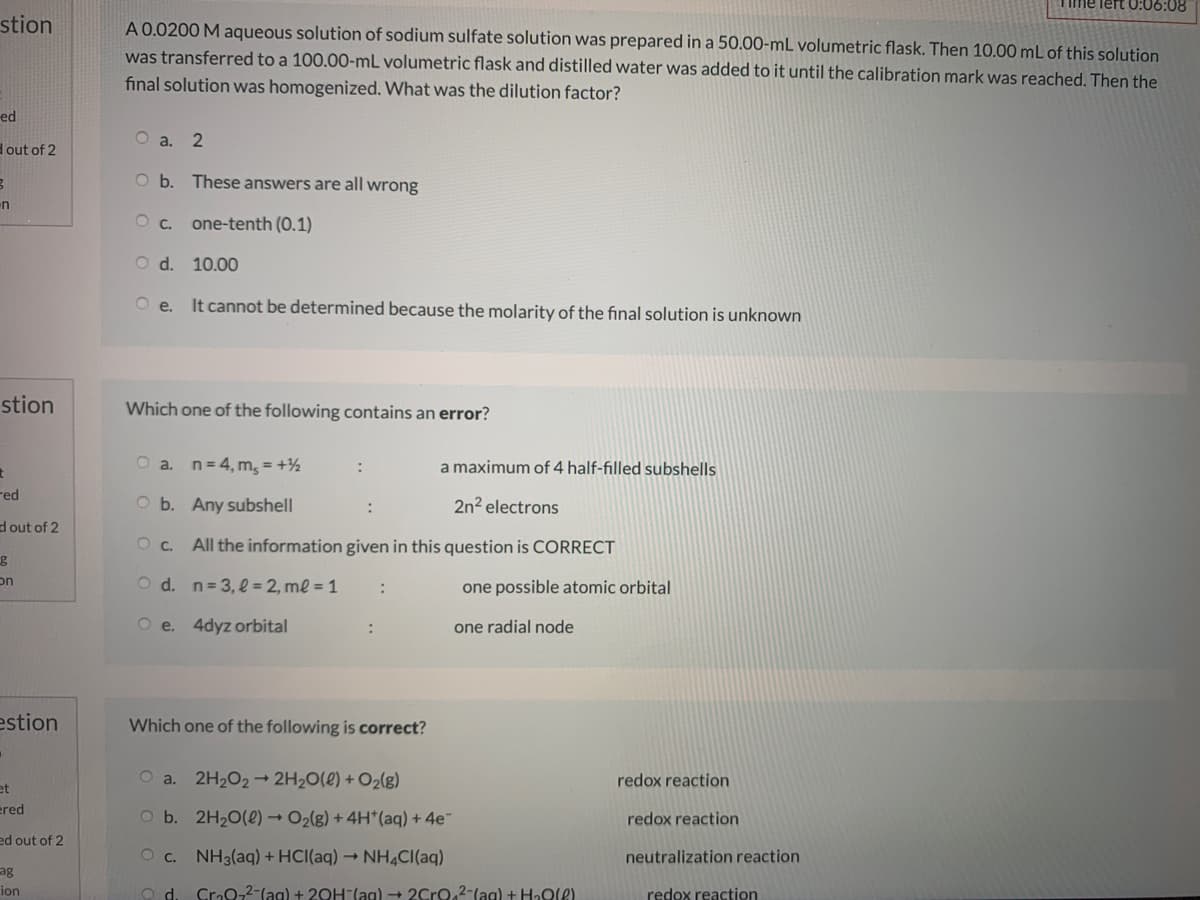
A 0.0200 M aqueous solution of sodium sulfate solution was prepared in a 50.00-mL volumetric flask. Then 10.00 mL of this solution
was transferred to a 100.00-mL volumetric flask and distilled water was added to it until the calibration mark was reached. Then the
final solution was homogenized. What was the dilution factor?
ed
O a. 2
Hout of 2
O b. These answers are all wrong
en
O c. one-tenth (0.1)
O d. 10.00
Oe.
It cannot be determined because the molarity of the final solution is unknown
stion
Which one of the following contains an error?
a. n= 4, m, = +½
a maximum of 4 half-filled subshells
red
O b. Any subshell
2n2 electrons
dout of 2
Oc.
All the information given in this question is CORRECT
O d. n= 3, l = 2, ml = 1
on
one possible atomic orbital
O e. 4dyz orbital
one radial node
estion
Which one of the following is correct?
O a. 2H2O2→ 2H2O(e) + O2(g)
redox reaction
et
ered
O b. 2H20(2) → O2(g) + 4H*(aq) + 4e
redox reaction
ed out of 2
O c. NH3(aq) + HCI(aq) → NH4CI(aq)
neutralization reaction
ag
Cro0-2-(ag) + 20H-(ag) → 2Cro,2-(ag) + HaOle)
redox reaction
ion
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning