A 0.2 m3 vessel contains 1265 kg of water at 90 ⁰C. Determine pressure, specific and total internal energy, specific and total enthalpy, and specific and total entropy
A 0.2 m3 vessel contains 1265 kg of water at 90 ⁰C. Determine pressure, specific and total internal energy, specific and total enthalpy, and specific and total entropy
Principles of Heat Transfer (Activate Learning with these NEW titles from Engineering!)
8th Edition
ISBN:9781305387102
Author:Kreith, Frank; Manglik, Raj M.
Publisher:Kreith, Frank; Manglik, Raj M.
Chapter5: Analysis Of Convection Heat Transfer
Section: Chapter Questions
Problem 5.5P: Evaluate the dimensionless groups hcD/k,UD/, and cp/k for water, n-butyl alcohol, mercury, hydrogen,...
Related questions
Question
A 0.2 m3 vessel contains 1265 kg of water at 90 ⁰C. Determine pressure, specific and total internal energy, specific and total enthalpy, and specific and total entropy
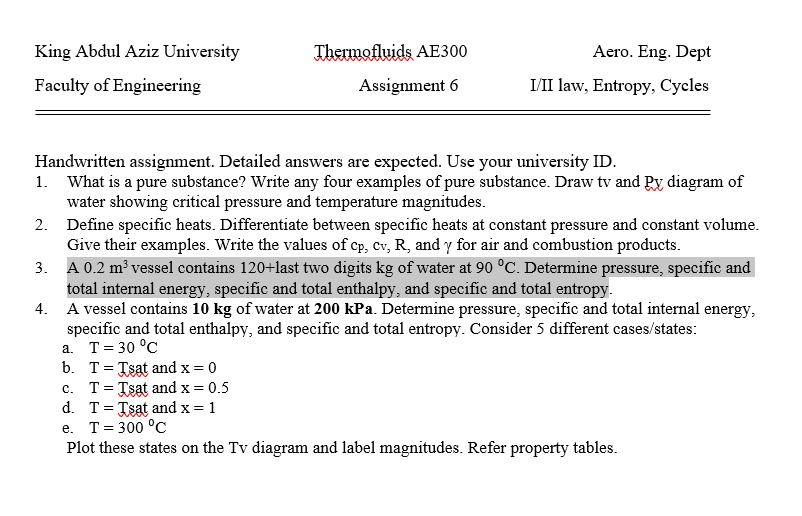
Transcribed Image Text:King Abdul Aziz University
Thermofluids AE300
Aero. Eng. Dept
Faculty of Engineering
Assignment 6
I/II law, Entropy, Cycles
Handwritten assignment. Detailed answers are expected. Use your university ID.
1. What is a pure substance? Write any four examples of pure substance. Draw tv and Py diagram of
water showing critical pressure and temperature magnitudes.
2. Define specific heats. Differentiate between specific heats at constant pressure and constant volume.
Give their examples. Write the values of cp, cv, R, and y for air and combustion products.
3. A 0.2 m³ vessel contains 120+last two digits kg of water at 90 °C. Determine pressure, specific and
total internal energy, specific and total enthalpy, and specific and total entropy.
4. A vessel contains 10 kg of water at 200 kPa. Determine pressure, specific and total internal energy,
specific and total enthalpy, and specific and total entropy. Consider 5 different cases/states:
a. T= 30 °C
b. T= Tsat and x = 0
c. T= Tsat and x = 0.5
d. T= Tsat and x = 1
e. T= 300 °C
Plot these states on the Tv diagram and label magnitudes. Refer property tables.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning