A 0.2160 g sample of primary standard grade HgO (MM = 216.59) was dissolved in 75 mL of an aqueous solution of KI. The liberated OH needed 22.13 mL of HCI to reach the end point. Calculate the molarity of the HCI solution. Tilrant Preliminary Rxn: HgO + 41+ H₂O→→→→→ Hgl2 + 2OH → H₂O + CI- Titration Rxn: OH + HCI
A 0.2160 g sample of primary standard grade HgO (MM = 216.59) was dissolved in 75 mL of an aqueous solution of KI. The liberated OH needed 22.13 mL of HCI to reach the end point. Calculate the molarity of the HCI solution. Tilrant Preliminary Rxn: HgO + 41+ H₂O→→→→→ Hgl2 + 2OH → H₂O + CI- Titration Rxn: OH + HCI
Chapter16: Applications Of Neutralization Titrations
Section: Chapter Questions
Problem 16.37QAP
Related questions
Question
100%
Please only do this typewritten, so everything is visible. I have a hard time reading handwritten solutions. i hope you understand. thank you. i will upvote
SKIP THIS IF YOU ALREADY DID THIS OR GET DOWNVOTE
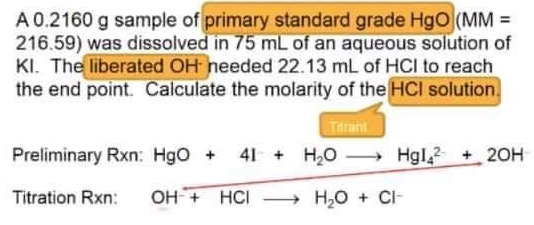
Transcribed Image Text:A 0.2160 g sample of primary standard grade HgO (MM =
216.59) was dissolved in 75 mL of an aqueous solution of
KI. The liberated OH needed 22.13 mL of HCI to reach
the end point. Calculate the molarity of the HCI solution.
Tilrant
Preliminary Rxn: HgO + 41+ H₂O→→→→→ Hgl2 + 2OH
→ H₂O + CI-
Titration Rxn: OH + HCI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax



Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning