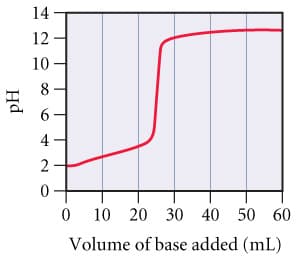
A 0.229-gg sample of an unknown monoprotic acid was titrated with 0.112 molL−1NaOHmolL−1NaOH and the resulting titration curve is shown in the picture Determine the molar mass of the acid. Express your answer using two significant figures. Determine pKapKa of the acid. Express your answer using one significant figure.
A 0.229-gg sample of an unknown monoprotic acid was titrated with 0.112 molL−1NaOHmolL−1NaOH and the resulting titration curve is shown in the picture Determine the molar mass of the acid. Express your answer using two significant figures. Determine pKapKa of the acid. Express your answer using one significant figure.
Chapter11: Organic Compounds: Alkanes
Section: Chapter Questions
Problem 11.3E
Related questions
Question
A 0.229-gg sample of an unknown monoprotic acid was titrated with 0.112 molL−1NaOHmolL−1NaOH and the resulting titration curve is shown in the picture
Determine the molar mass of the acid.
Express your answer using two significant figures.
Determine pKapKa of the acid.
Express your answer using one significant figure.
Transcribed Image Text:Hd
14-
12
10
8
6
4
2
0
10 20 30 40 50 60
Volume of base added (mL)
0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

