A 0.5-kg block of a pure material is heated from 0°C to 93.75°C by the addition of 6 kJ of energy. Calculate its specific heat and identify the substance of which it is most likely composed. (2 acceptable answers) Use the Specific heat table provided. (Work on Paper Required) Substances Specific heat (c) Solids J/kg · °C Aluminum 900 Asbestos 800 Concrete, granite (average) 840 Copper 387 Glass 840 Gold 129 Human body (average at 37 °C) 3500 Ice (average, -50°C to 0°C) 2090 Iron, steel 452 Lead 128 Silver 235 Wood 1700 Liquids Benzene 1740 Ethanol 2450
A 0.5-kg block of a pure material is heated from 0°C to 93.75°C by the addition of 6 kJ of energy. Calculate its specific heat and identify the substance of which it is most likely composed. (2 acceptable answers) Use the Specific heat table provided. (Work on Paper Required) Substances Specific heat (c) Solids J/kg · °C Aluminum 900 Asbestos 800 Concrete, granite (average) 840 Copper 387 Glass 840 Gold 129 Human body (average at 37 °C) 3500 Ice (average, -50°C to 0°C) 2090 Iron, steel 452 Lead 128 Silver 235 Wood 1700 Liquids Benzene 1740 Ethanol 2450
Physics for Scientists and Engineers, Technology Update (No access codes included)
9th Edition
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter20: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 20.10OQ: A 100-g piece of copper, initially at 95.0C, is dropped into 200 g of water contained in a 280-g...
Related questions
Question
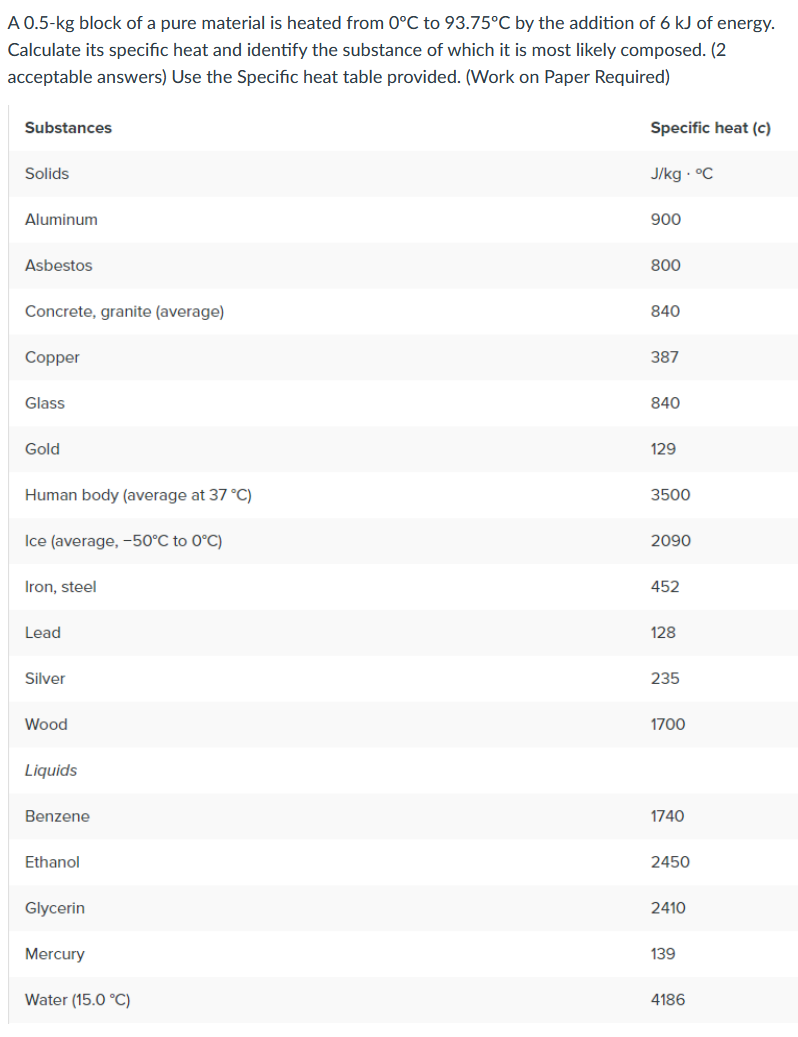
Transcribed Image Text:A 0.5-kg block of a pure material is heated from 0°C to 93.75°C by the addition of 6 kJ of energy.
Calculate its specific heat and identify the substance of which it is most likely composed. (2
acceptable answers) Use the Specific heat table provided. (Work on Paper Required)
Substances
Specific heat (c)
Solids
J/kg · °C
Aluminum
900
Asbestos
800
Concrete, granite (average)
840
Copper
387
Glass
840
Gold
129
Human body (average at 37 °C)
3500
Ice (average, -50°C to 0°C)
2090
Iron, steel
452
Lead
128
Silver
235
Wood
1700
Liquids
Benzene
1740
Ethanol
2450
Glycerin
2410
Mercury
139
Water (15.0 °C)
4186
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning