) A 0.70 μL sample of an equal volume mixture of 2-pentanone and 1-nitropropane is injected into a gas chromatograph. he densities of these compounds are 0.8124 g/mL for 2-pentanone and 1.0221 g/mL for 1-nitropropane. What mass of each compound was injected? lass of 2-pentanone = mg mg ass of 1-nitropropane = 49 ) The peak areas produced on this injection were 1402 units for 2-pentanone and 1181 units for 1-nitropropane. alculate the response factor for each compound as area per mg. -pentanone: 4.0 units/mg units/mg -nitropropane: 4.0 An unknown mixture of these two components produces peak areas of 1515 units (2-pentanone) and 1446 units (1- itropropane). se these areas and the response factors above to determine the weight % of the components in the unknown sample. -pentanone: 4.0 -nitropropane: 4.0 % %
) A 0.70 μL sample of an equal volume mixture of 2-pentanone and 1-nitropropane is injected into a gas chromatograph. he densities of these compounds are 0.8124 g/mL for 2-pentanone and 1.0221 g/mL for 1-nitropropane. What mass of each compound was injected? lass of 2-pentanone = mg mg ass of 1-nitropropane = 49 ) The peak areas produced on this injection were 1402 units for 2-pentanone and 1181 units for 1-nitropropane. alculate the response factor for each compound as area per mg. -pentanone: 4.0 units/mg units/mg -nitropropane: 4.0 An unknown mixture of these two components produces peak areas of 1515 units (2-pentanone) and 1446 units (1- itropropane). se these areas and the response factors above to determine the weight % of the components in the unknown sample. -pentanone: 4.0 -nitropropane: 4.0 % %
Chapter5: Errors In Chemical Analyses
Section: Chapter Questions
Problem 5.12QAP
Related questions
Question
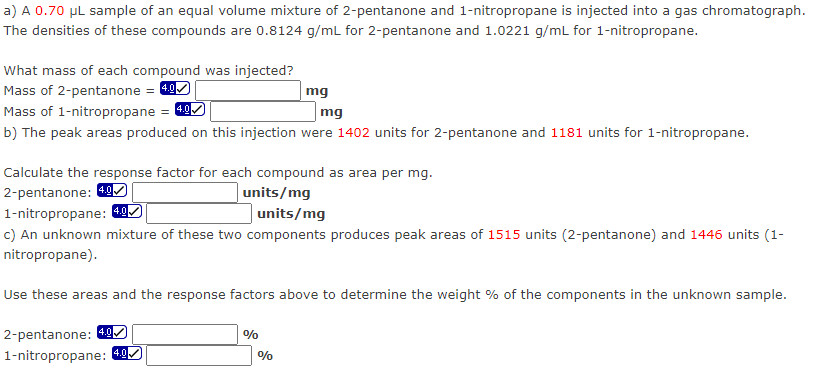
Transcribed Image Text:a) A 0.70 µL sample of an equal volume mixture of 2-pentanone and 1-nitropropane is injected into a gas chromatograph.
The densities of these compounds are 0.8124 g/mL for 2-pentanone and 1.0221 g/mL for 1-nitropropane.
What mass of each compound was injected?
Mass of 2-pentanone = 4.0
mg
mg
Mass of 1-nitropropane = 4.9
b) The peak areas produced on this injection were 1402 units for 2-pentanone and 1181 units for 1-nitropropane.
Calculate the response factor for each compound as area per mg.
2-pentanone: 4.0
units/mg
units/mg
1-nitropropane: 4.0
c) An unknown mixture of these two components produces peak areas of 1515 units (2-pentanone) and 1446 units (1-
nitropropane).
Use these areas and the response factors above to determine the weight % of the components in the unknown sample.
2-pentanone: 4.0
1-nitropropane: 4.0
%
%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole