A 0.885 mol sample of He gas is confined in a 22.3 liter container at 34.3 °C. If 0.885 mol of N2 is substituted for the 0.885 mol of He, holding the volume and temperature constant, the average kinetic energy will decrease increase remain the same not enough information to answer the question
A 0.885 mol sample of He gas is confined in a 22.3 liter container at 34.3 °C. If 0.885 mol of N2 is substituted for the 0.885 mol of He, holding the volume and temperature constant, the average kinetic energy will decrease increase remain the same not enough information to answer the question
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter5: Gases
Section: Chapter Questions
Problem 70QAP: Given that 1.00 mol of neon and 1.00 mol of hydrogen chloride gas are in separate containers at the...
Related questions
Question
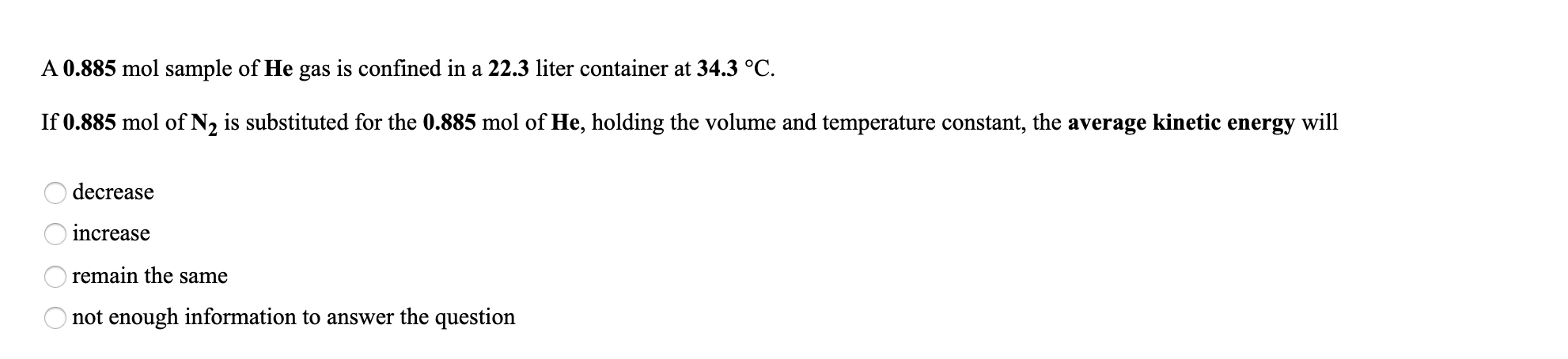
Transcribed Image Text:A 0.885 mol sample of He gas is confined in a 22.3 liter container at 34.3 °C.
If 0.885 mol of N2 is substituted for the 0.885 mol of He, holding the volume and temperature constant, the average kinetic energy will
decrease
increase
remain the same
not enough information to answer the question
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning