A 10 kg of ice at 0 °C is mixed with 4 kg of steam at 100 °C in a thermally isolated container and allowed to reach thermal equilibrium. What is the amount of heat needed to melt the ice? The latent heats of fusion and vaporization of water is 334000 J/kg and 2256000 J/kg, respectively and the specific heat of liquid water is 4186 J/kg.K.
A 10 kg of ice at 0 °C is mixed with 4 kg of steam at 100 °C in a thermally isolated container and allowed to reach thermal equilibrium. What is the amount of heat needed to melt the ice? The latent heats of fusion and vaporization of water is 334000 J/kg and 2256000 J/kg, respectively and the specific heat of liquid water is 4186 J/kg.K.
College Physics
10th Edition
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Raymond A. Serway, Chris Vuille
Chapter11: Energy In Thermal Processes
Section: Chapter Questions
Problem 15P: What mass of water at 25.0C must be allowed to conic to thermal equilibrium with a 1.85-kg cube of...
Related questions
Question
A 10 kg of ice at 0 °C is mixed with 4 kg of steam at 100 °C in a thermally isolated container and allowed to reach thermal equilibrium. What is the amount of heat needed to melt the ice? The latent heats of fusion and vaporization of water is 334000 J/kg and 2256000 J/kg, respectively and the specific heat of liquid water is 4186 J/kg.K.
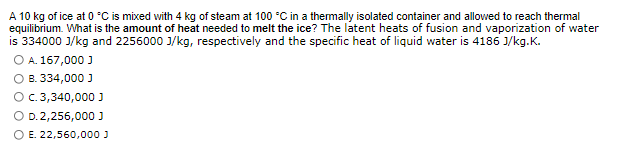
Transcribed Image Text:A 10 kg of ice at 0 °C is mixed with 4 kg of steam at 100 °C in a thermally isolated container and allowed to reach thermal
equilibrium. What is the amount of heat needed to melt the ice? The latent heats of fusion and vaporization of water
is 334000 J/kg and 2256000 J/kg, respectively and the specific heat of liquid water is 4186 J/kg.K.
O A. 167,000 J
O B. 334,000 J
O c. 3,340,000 J
O D.2,256,000 J
O E. 22,560,000 J
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning


College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning
