A 2.75-g copper coin at 20.0°C drops 60.0 m to the ground. (a) Assuming 55.0% of the change in gravitational potential energy of the coin-Earth system goes into increasing the internal energy of the coin, determine the coin's final temperature. °C (b) Does the result depend on the mass of the coin? Yes No Explain your answer.
A 2.75-g copper coin at 20.0°C drops 60.0 m to the ground. (a) Assuming 55.0% of the change in gravitational potential energy of the coin-Earth system goes into increasing the internal energy of the coin, determine the coin's final temperature. °C (b) Does the result depend on the mass of the coin? Yes No Explain your answer.
College Physics
10th Edition
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Raymond A. Serway, Chris Vuille
Chapter11: Energy In Thermal Processes
Section: Chapter Questions
Problem 5P: A 3.00-g copper coin at 25.0C drops 50.0 m to the ground. (a) Assuming 60.0% of the change in...
Related questions
Question
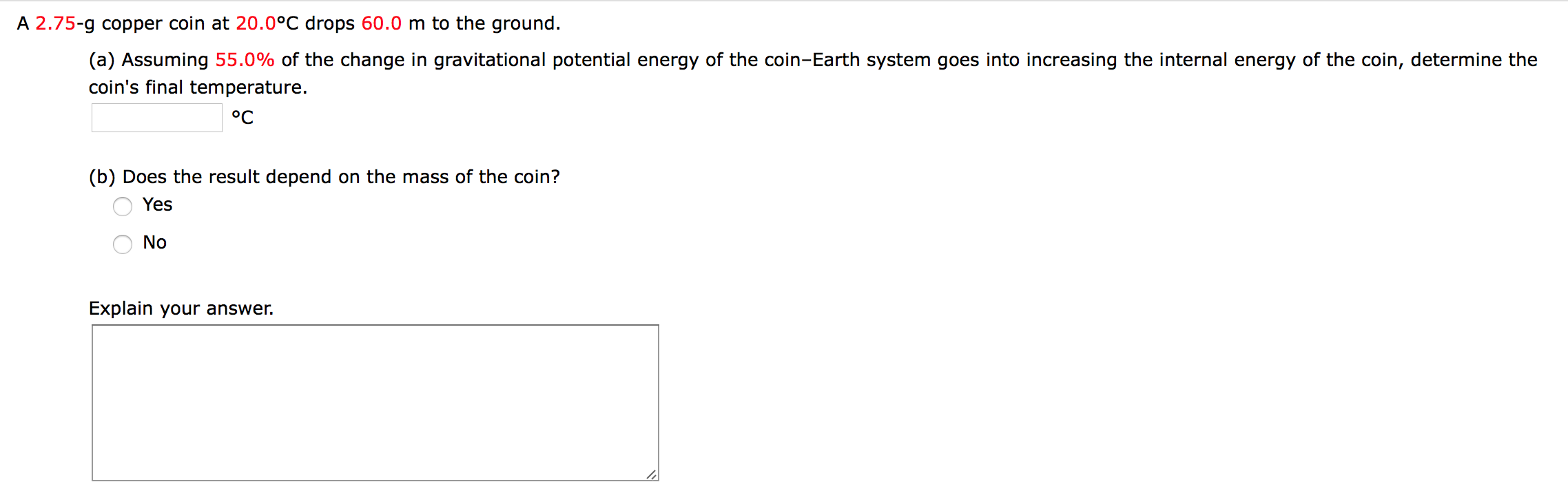
Transcribed Image Text:A 2.75-g copper coin at 20.0°C drops 60.0 m to the ground.
(a) Assuming 55.0% of the change in gravitational potential energy of the coin-Earth system goes into increasing the internal energy of the coin, determine the
coin's final temperature.
°C
(b) Does the result depend on the mass of the coin?
Yes
No
Explain your answer.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning