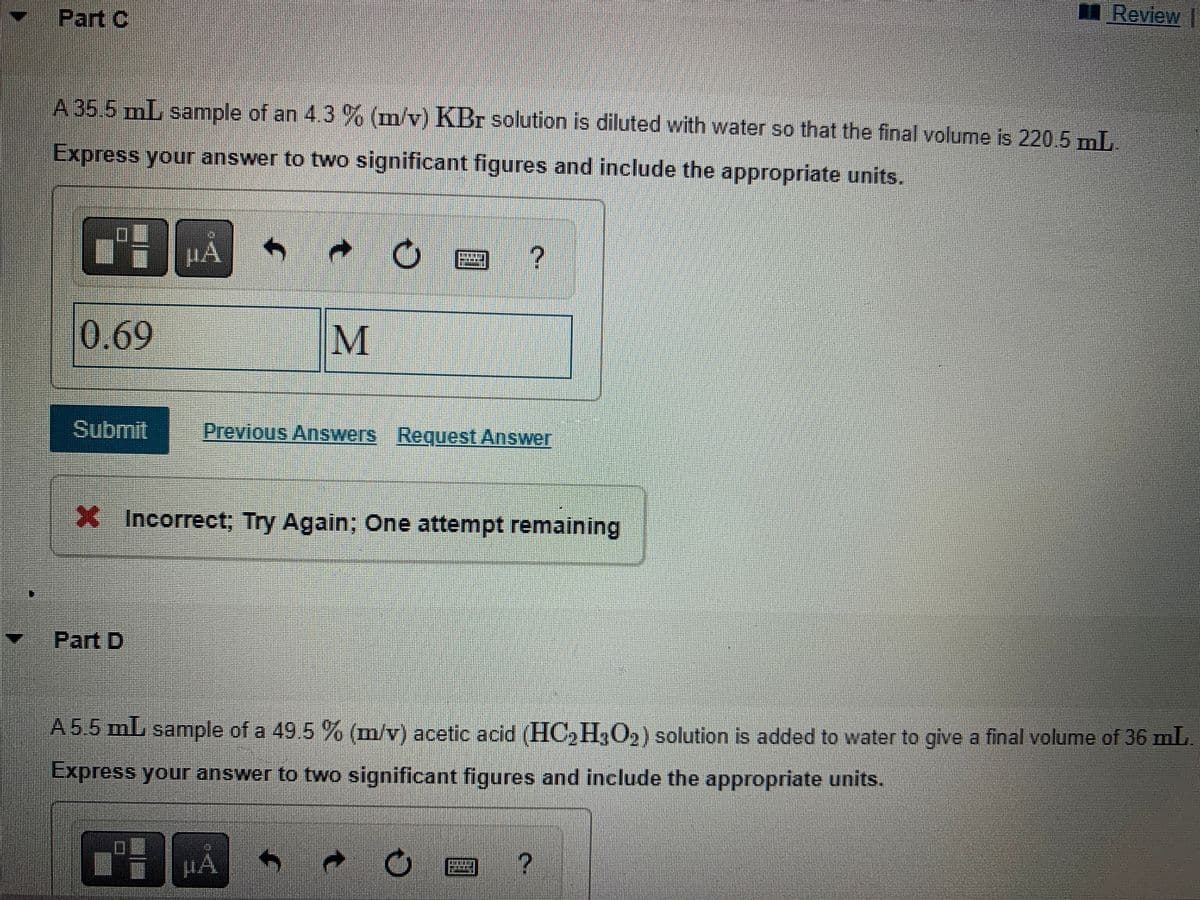
A 35.5 mL sample of an 4.3 % (m/v) KBr solution is diluted with water so that the final volume is 220.5 mL. Express your answer to two significant figures and include the appropriate units.
A 35.5 mL sample of an 4.3 % (m/v) KBr solution is diluted with water so that the final volume is 220.5 mL. Express your answer to two significant figures and include the appropriate units.
Chapter7: Solutions And Colloids
Section: Chapter Questions
Problem 7.44E
Related questions
Question
Calculate the final calculation of each of the following.
Transcribed Image Text:Part C
A 35.5 mL sample of an 4.3 % (m/v) KBr solution is diluted with water so that the final volume is 220.5 mL.
Express your answer to two significant figures and include the appropriate units.
7 µÅ h
0.69
Submit
Part D
t
M
Previous Answers Request Answer
X Incorrect; Try Again; One attempt remaining
μA
?
A 5.5 mL sample of a 49.5 % (m/v) acetic acid (HC₂H3O2) solution is added to water to give a final volume of 36 mL.
Express your answer to two significant figures and include the appropriate units.
C
Review |
圖?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning



Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning