A 50.00-mL sample of 0.0750 M HCN was titrated with 0.3995 M NaOH. What is the pH at the equivalence point at 25°C? Ka for HCN is 6.2x10-10 at 25°C. 9.11 10.0 O 11.0 8.11
A 50.00-mL sample of 0.0750 M HCN was titrated with 0.3995 M NaOH. What is the pH at the equivalence point at 25°C? Ka for HCN is 6.2x10-10 at 25°C. 9.11 10.0 O 11.0 8.11
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter14: Acid- Base Equilibria
Section: Chapter Questions
Problem 110CP: A 0.400-M solution of ammonia was titrated with hydrochloric acid to the equivalence point, where...
Related questions
Question
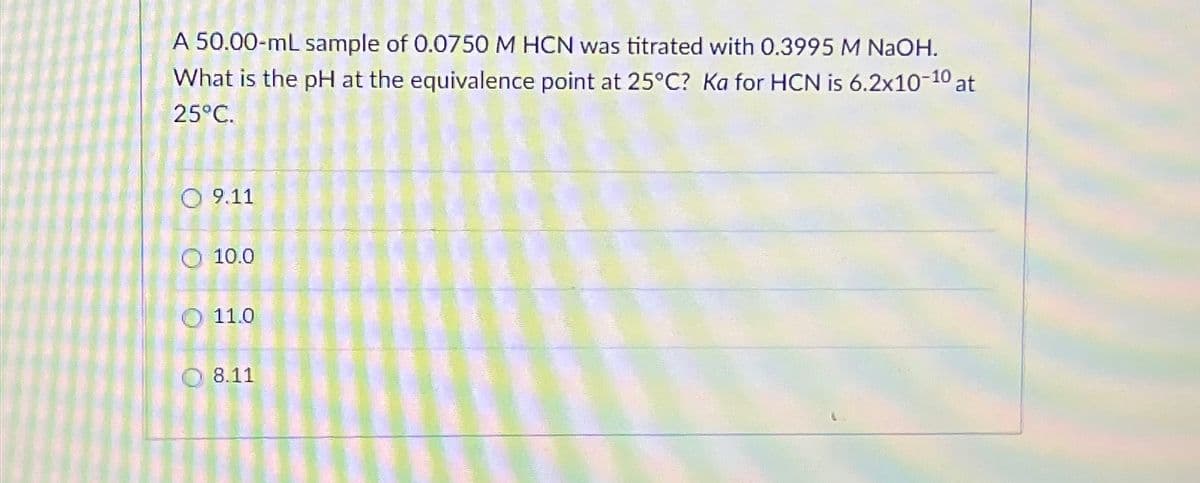
Transcribed Image Text:A 50.00-mL sample of 0.0750 M HCN was titrated with 0.3995 M NaOH.
What is the pH at the equivalence point at 25°C? Ka for HCN is 6.2x10-10 at
25°C.
9.11
10.0
O
11.0
8.11
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning