A 575-mL sample of nitrogen has a pressure of 720 Torr at 27oC. a. What will be the new volume if the temperature is raised to 77oC, while the pressure is maintained (i.e., 720 Torr)? ____________ mL b. What will be the new volume if the pressure is raised to 780 Torr, while the original temperature is maintained (i.e., 27oC)? ____________ mL c. What will be the new volume if the pressure is raised to 780 Torr and the temperature is raised to 77oC?
A 575-mL sample of nitrogen has a pressure of 720 Torr at 27oC.
a. What will be the new volume if the temperature is raised to 77oC,
while the pressure is maintained (i.e., 720 Torr)? ____________ mL
b. What will be the new volume if the pressure is raised to 780 Torr,
while the original temperature is maintained (i.e., 27oC)? ____________ mL
c. What will be the new volume if the pressure is raised to 780 Torr
and the temperature is raised to 77oC?
According to ideal gas law,
PV = nRT
where P = pressure
V = volume
n = moles
R = gas constant = 0.0821 atm.L/K.mol
T = temperature in K = T in °C + 273
a) Since the pressure is kept constant and also amount of gas is not changing. Hence P and n are constant.
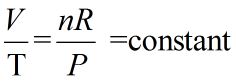
Hence we can say
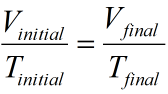
where Vinitial = initial volume = 575 mL and Tinitial = initial temperature in K = 273 + 27 = 300 K
and Vfinal = final volume and Tfinal = final temperature in K = 273 + 77 = 350 K
Hence substituting the values we get
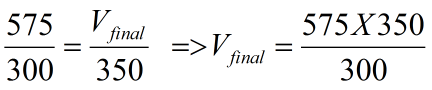
=> Vfinal = 670.8 mL approx = the new volume
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 6 images









