A bowl of hot soup and a glass of ice water are left in a room. Eventually the soup and th- O Heat energy went from the ice water to the soup and from the soup to the room. O Heat energy went from the soup to the room and from the room into the ice water. O The heat of the soup and the cold of the water were exchanged, cancelling each other out. O The low temperature of the water was transferred to the room and from the room to the soup.
A bowl of hot soup and a glass of ice water are left in a room. Eventually the soup and th- O Heat energy went from the ice water to the soup and from the soup to the room. O Heat energy went from the soup to the room and from the room into the ice water. O The heat of the soup and the cold of the water were exchanged, cancelling each other out. O The low temperature of the water was transferred to the room and from the room to the soup.
Physics for Scientists and Engineers: Foundations and Connections
1st Edition
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Katz, Debora M.
Chapter19: Temperature, Thermal Expansion And Gas Laws
Section: Chapter Questions
Problem 62PQ
Related questions
Question
A bowl of hot soup and a glass of ice water are left in a room. Eventually the soup and the water become the same temperature as their surroundings. Which of the following explains the flow of heat that led to this final outcome?
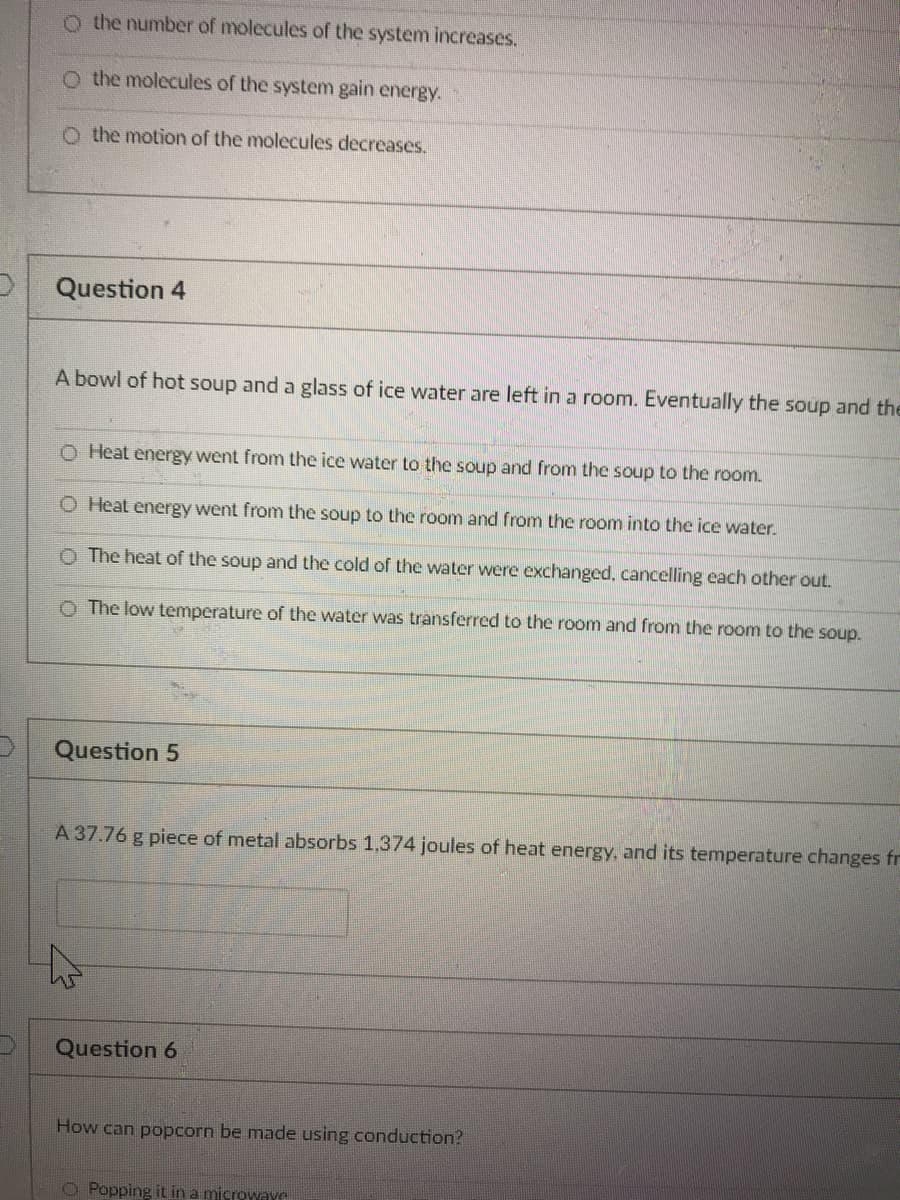
Transcribed Image Text:o the number of molecules of the system increases.
O the molecules of the system gain energy.
O the motion of the molecules decreases,
Question 4
A bowl of hot soup and a glass of ice water are left in a room. Eventually the soup and the
O Heat energy went from the ice water to the soup and from the soup to the room.
O Heat energy went from the soup to the room and from the room into the ice water.
The heat of the soup and the cold of the water were exchanged, cancelling each other out.
O The low temperature of the water was transferred to the room and from the room to the soup.
Question 5
A 37.76 g piece of metal absorbs 1,374 joules of heat energy, and its temperature changes fr
Question 6
How can popcorn be made using conduction?
O Popping it in a microwave
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning