A brick of a certain metal has area on top of 3.34 cm x 7.33 cm. lIt has mass 791 g. How high is the brick in cm? The metal has density of 11.8 g/cm3 2.54 cm = 1 inch Ixhxw = V V = m/d You need to combine the above equations; this is a typical math skill you need in chemistry.
A brick of a certain metal has area on top of 3.34 cm x 7.33 cm. lIt has mass 791 g. How high is the brick in cm? The metal has density of 11.8 g/cm3 2.54 cm = 1 inch Ixhxw = V V = m/d You need to combine the above equations; this is a typical math skill you need in chemistry.
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 34E: The beakers shown below have different precisions. a. Label the amount of water in each of the three...
Related questions
Question
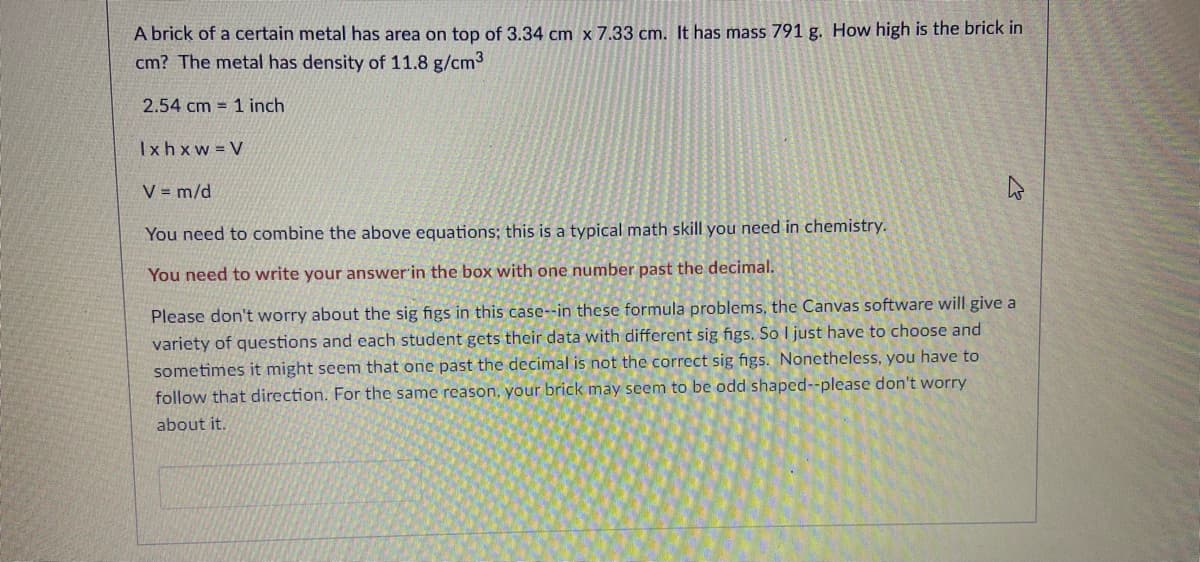
Transcribed Image Text:A brick of a certain metal has area on top of 3.34 cm x 7.33 cm. It has mass 791 g. How high is the brick in
cm? The metal has density of 11.8 g/cm3
2.54 cm = 1 inch
Ixhxw V
V = m/d
You need to combine the above equations; this is a typical math skill you need in chemistry.
You need to write your answer'in the box with one number past the decimal.
Please don't worry about the sig figs in this case--in these formula problems, the Canvas software will give a
variety of questions and each student gets their data with different sig figs. So I just have to choose and
sometimes it might seem that one past the decimal is not the correct sig figs. Nonetheless, you have to
follow that direction. For the same reason, your brick may seem to be odd shaped--please don't worry
about it.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning