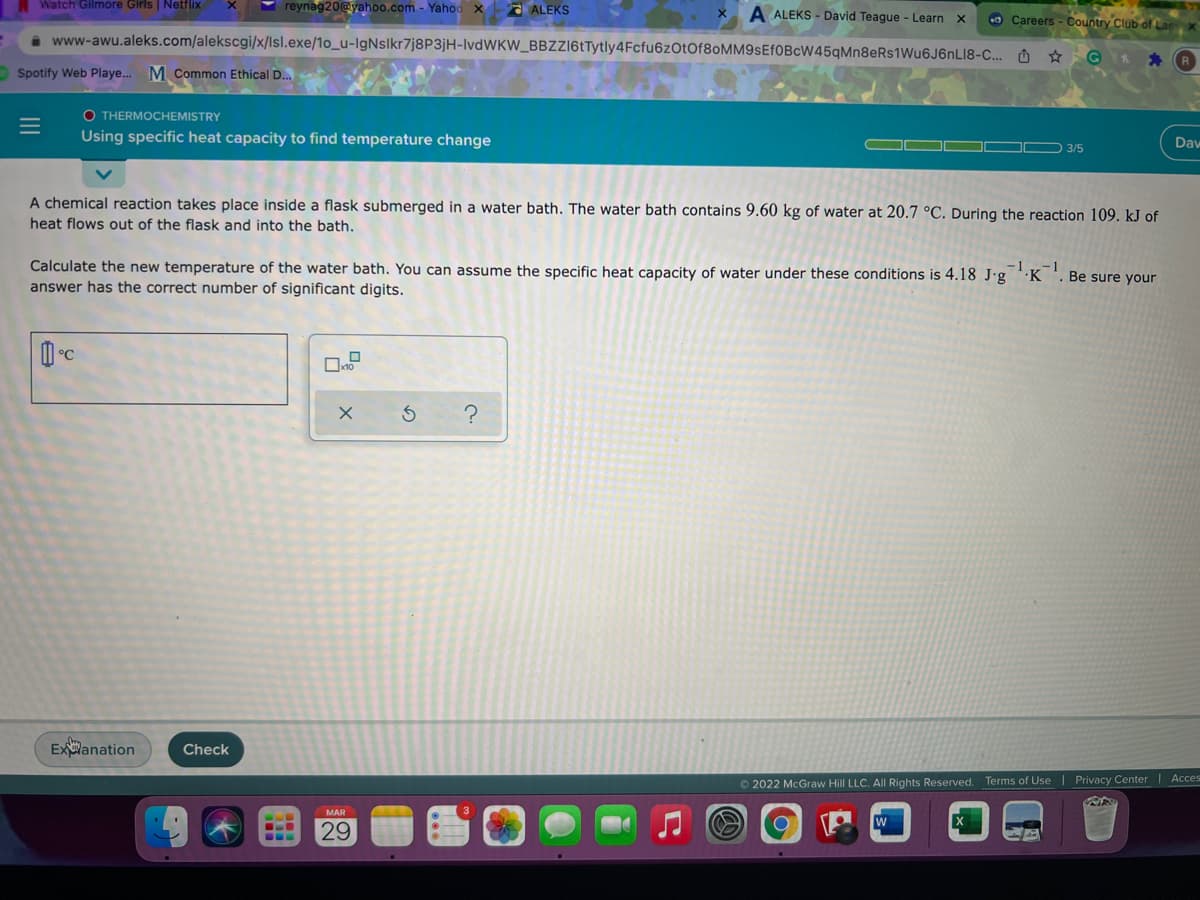
A chemical reaction takes place inside a flask submerged in a water bath. The water bath contains 9.60 kg of water at 20.7 °C. During the reaction 109. kJ of heat flows out of the flask and into the bath. Calculate the new temperature of the water bath. You can assume the specific heat capacity of water under these conditions is 4.18 J-g 'K answer has the correct number of significant digits. -1 Be sure your °C
A chemical reaction takes place inside a flask submerged in a water bath. The water bath contains 9.60 kg of water at 20.7 °C. During the reaction 109. kJ of heat flows out of the flask and into the bath. Calculate the new temperature of the water bath. You can assume the specific heat capacity of water under these conditions is 4.18 J-g 'K answer has the correct number of significant digits. -1 Be sure your °C
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter13: The Chemistry Of Solutes And Solutions
Section: Chapter Questions
Problem 83QRT
Related questions
Question
Transcribed Image Text:Watch Gilmore Girls | Netflix
reynag20@yahoo.com - Yahoo x
a ALEKS
A ALEKS - David Teague - Learn x
O Careers - Country Club of Lan
www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IvdWKW_BBZZ16tTytly4Fcfu6zOtOf8oMM9sEf0BcW45qMn8eRs1Wu6J6nLI8-C.. O ☆
O Spotify Web Playe... M Common Ethical D.
O THERMOCHEMISTRY
Using specific heat capacity to find temperature change
Daw
O 3/5
A chemical reaction takes place inside a flask submerged in a water bath. The water bath contains 9.60 kg of water at 20.7 °C. During the reaction 109. kJ of
heat flows out of the flask and into the bath.
Calculate the new temperature of the water bath. You can assume the specific heat capacity of water under these conditions is 4.18 J'g 'K
answer has the correct number of significant digits.
-1
Be sure your
°C
ロ
Exlanation
Check
© 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Acces
29
の
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co