A chemist adds 1.20 L of a 0.67M aluminum sulfate (AL solution to a reaction flask. Calculate the millimoles of aluminum (Al₂(SO4)3) sulfate the chemist has added to the flask. Be sure your answer has the correct number of significant digits.
A chemist adds 1.20 L of a 0.67M aluminum sulfate (AL solution to a reaction flask. Calculate the millimoles of aluminum (Al₂(SO4)3) sulfate the chemist has added to the flask. Be sure your answer has the correct number of significant digits.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter2: Chemical Compounds
Section: Chapter Questions
Problem 105QRT
Related questions
Question
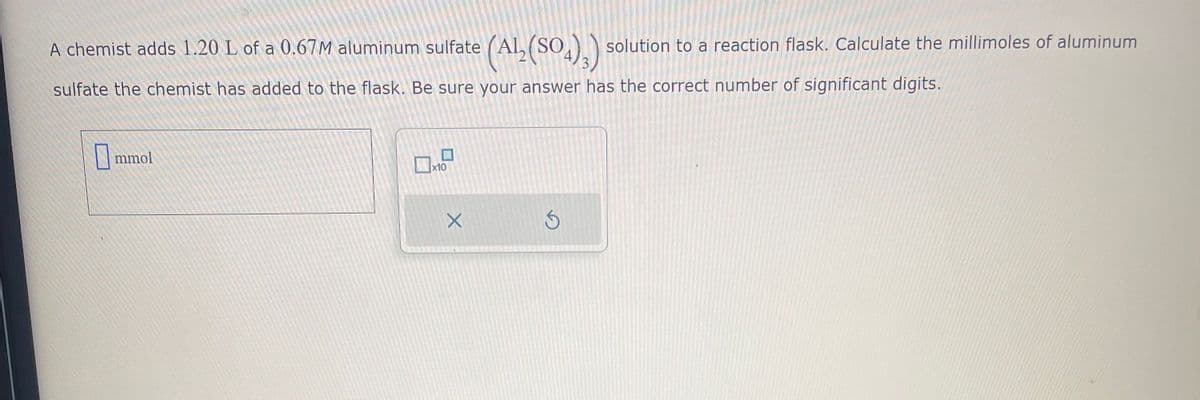
Transcribed Image Text:A chemist adds 1.20 L of a 0.67M aluminum sulfate (Al₂(SO4)3) solution to a reaction flask. Calculate the millimoles of aluminum
sulfate the chemist has added to the flask. Be sure your answer has the correct number of significant digits.
mmol
x10
X
S
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning