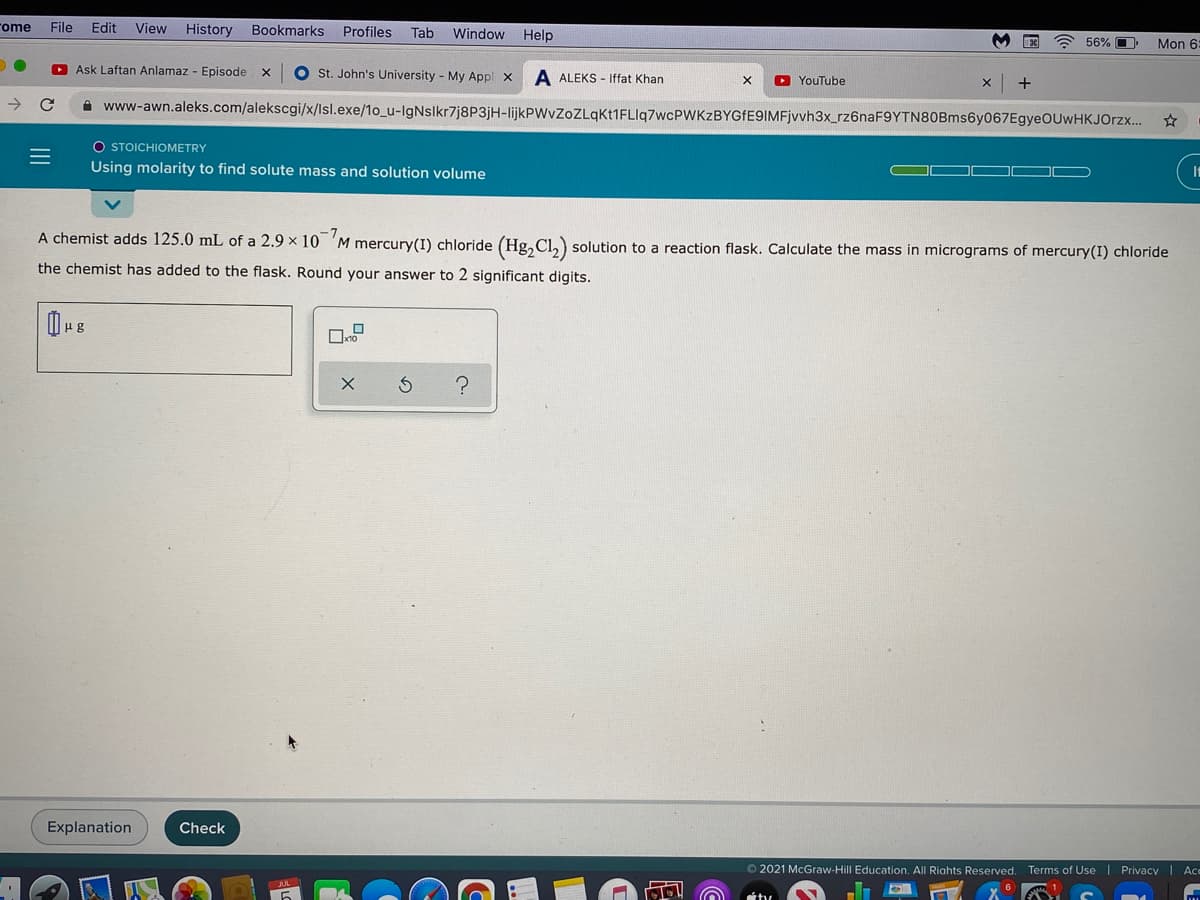
A chemist adds 125.0 mL of a 2.9 x 10 'M mercury(I) chloride (Hg, Cl,) solution to a reaction flask. Calculate the mass in micrograms of mercury(I) chloride the chemist has added to the flask. Round your answer to 2 significant digits.
A chemist adds 125.0 mL of a 2.9 x 10 'M mercury(I) chloride (Hg, Cl,) solution to a reaction flask. Calculate the mass in micrograms of mercury(I) chloride the chemist has added to the flask. Round your answer to 2 significant digits.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter2: Atoms Molecules And Ions
Section: Chapter Questions
Problem 11PS: Marie Curie was born in Poland but studied and carried out her research in Paris. In 1903, she...
Related questions
Question
Transcribed Image Text:ome
File
Edit
View History Bookmarks
Profiles
Tab
Window Help
56% O
Mon 6
O Ask Laftan Anlamaz - Episode x
O St. John's University - My Appl x
A ALEKS - Iffat Khan
O YouTube
+
->
A www-awn.aleks.com/alekscgi/x/lsl.exe/1o_u-lgNslkr7j8P3jH-lijkPWvZoZLqkt1FLIq7wcPWKzBYGfE9IMFjvvh3x_rz6naF9YTN80Bms6y067EgyeOUwHKJOrzx.
O STOICHIOMETRY
Using molarity to find solute mass and solution volume
It
A chemist adds 125.0 mL of a 2.9 x 10 'M mercury(I) chloride (Hg, Cl,) solution to a reaction flask. Calculate the mass in micrograms of mercury(I) chloride
the chemist has added to the flask. Round your answer to 2 significant digits.
με
Explanation
Check
© 2021 McGraw-Hill Education. All Riahts Reserved. Terms of Use | Privacy I Ace
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning